Shoulder:Glenohumeral Arthritis/Anatomic Shoulder Arthroplasty
Contents
Bullet Points
Key words
History
This subsection does not exist. You can ask for it to be created, but consider checking the search results below to see whether the topic is already covered.
Anecdotes
This subsection does not exist. You can ask for it to be created, but consider checking the search results below to see whether the topic is already covered.
Biomechanics
- REDIRECT [[1]]
Introduction
Anatomy is key to successfully reproduce patient’s physiologic joint kinematics. By virtue of its mobility, the glenohumeral joint is predisposed to instability. One factor affecting stability is the radius of curvature mismatch between the humeral head and glenoid. Further, only 20 to 30% of the humeral head is in contact with the glenoid.[1] The rotator cuff acts as an essential dynamic stabilizing force centering the humeral in the mid-portion of range of motion and is crucial for anatomic total shoulder arthroplasty to be effective.[2] The supraspinatus helps to center the humeral head against the force of the deltoid in lower degrees of abduction, while the infraspinatus and teres minor help to clear the greater tuberosity under the coraco-acromial arch when the arm is moved in abduction and external rotation.[2][3] Lastly, even though the shoulder is not a weight-bearing joint, joint reaction forces as high as 2,4 times body weight have been reported during shoulder rehabilitation.[4]
Humeral head
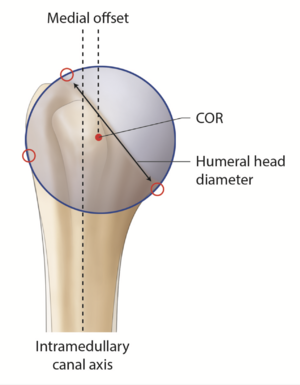
Proximal humerus anatomy is subject to great variability, which is further significantly modified by arthritic changes.[5][6] As anatomic total shoulder arthroplasty can restore physiologic shoulder kinetics, a thorough knowledge of normal anatomy appears mandatory as one cannot simply rely on perioperative measures (Figure).[7] The non-arthritic humeral head has a mean three-dimensional measured diameter of 46.2 + 5.4 mm (range, 37.1 to 56.9 mm) and a humeral height of approximately 19 mm (Figure).[8][9][10][11][12]
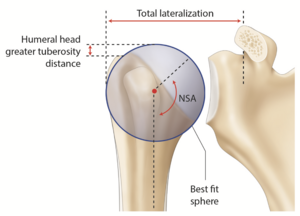
The osteoarthritic head is flattened and widened with a mean diameter of 59 ± 9 mm.[5] The humeral head has the particularity to be elliptic in the periphery and become spherical in its central part, meaning that the cut surface will be about 2 mm larger from medial to lateral than from anterior to posterior.[12] While spherical humeral head implants are mainly used in shoulder arthroplasty, elliptic implants have been proposed to reproduce anatomy and theoretically improve the rotational range of motion. The ratio between humeral head size and height is relatively constant.[13] The highest point of the humeral head lies 8 + 3.2 mm above the greater tuberosity (Figure).[12]
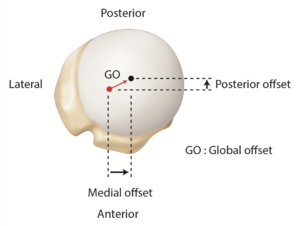
Lastly, relative to the humeral canal, the head has a posterior and medial offset of 0.35 to 2.6 mm and 5.6 to 9.7 mm, respectively (Figures).[14][15]
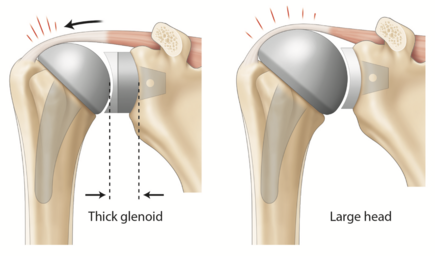
These parameters are helpful to select the appropriate humeral head implant, as this crucial step will ultimately determine the joint center of rotation. However, current biomechanical data does not support significant superiority of the elliptic design over the spherical one regarding the range of motion in internal and external rotation.[16] Terrier et al. illustrated in a numerical shoulder model that a 5 mm malposition of the humeral head implant resulted in impingement or subluxation for an inferior or superior shift, respectively. Both resulted in increased stress on the cement mantle.[17] While joint center of rotation can be determined three-dimensionally by a best-fit sphere using preserved non-articular landmarks, this technique has been translated to a two-dimensional process to allow intraoperative as well as postoperative radiographic evaluation (Figures).[6][18]
However, there is no consensus on cut-off values for joint center of rotation modification, as values as low as 2.5 mm can have been reported to impact impingement free range of motion.[19] Further, if the humeral head is implanted 5 mm too high in regard to the tuberosity, shoulder function will not solely be impaired by a 4 mm decrease in infraspinatus and subscapularis lever arms but also by the tight inferior capsule.[20] Cadaveric studies revealed that an increased humeral component sizing (commonly called “overstuffing”) would modify the center of rotation and add stress to the rotator cuff (Figure). Overstuffing not only decreases shoulder range of motion but also changes rotator cuff lever arm exposing patients to the potential risk of secondary cuff failure.[21][22] Restoring physiologic soft-tissue tension will provide stability and prevent complications such as aseptic loosening and osteolysis induced by stress shielding.[23] Lastly, controversy exists regarding the superiority of resurfacing humeral head over stemmed implants to reproduce physiological shoulder biomechanics.[6][24]
Neck shaft angle
The mean neck-shaft angle or inclination of the proximal humerus is approximately 135 degrees but varies between 115 and 148 degrees (Figure). A study of 2058 humeri by Jeong et al. note that 22% are either < 130 degrees or > 140 degrees.[25] Thus, fixed neck shaft angle humeral stems rely on surgeons to adapt their surgical techniques to accommodate patient anatomy. Modern modular systems provide centered and eccentric humeral heads as well as multiple neck-shaft angle options.
Humeral torsion
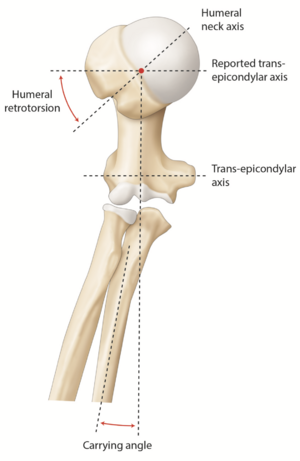
Humeral head torsion is important in anatomic total shoulder arthroplasty as it directly affects joint center of rotation and thereby influences mobility in external rotation and shoulder stability.[26][27][28] A cadaveric study by Pearl and Volk reported a mean humeral retrotorsion of 29.8 degrees with a 95% confidence interval of 7 to 52 degrees (Figure).[29] While they used the trochlear axis as a reference, other reported values were based on the transepicondylar axis (which differs from 3 to 8 degrees). Furthermore, current systems use a jig aligned on the forearm as a reference, in this case, a 10 to 15 degrees (carrying angle) must be added to the reported values (Figure). When using a stem with lateral fins, another reliable landmark is to place it 12 + 4 mm behind the bicipital groove.[30] It should, however, be emphasized that the groove rotates about 16 + 7 degrees and appears therefore as an unsuitable landmark in fracture or posttraumatic cases.[31] Lastly, Raniga et al. reported that in Walch B type glenoids, humeral retrotorsion is significantly lower compared to none-arthritic shoulders (14 + 9 degrees vs. 36 ± 12 degrees, p<0.001), suggesting a potential correlation between humeral retrotorsion and glenoid retroversion.[32]
Glenohumeral offset
Osteoarthritis results in loss of glenohumeral offset secondary to humeral and glenoid bone wear. While glenohumeral offset is subject to inter-person-variability, a diminished glenohumeral offset implies altered deltoid and rotator cuff moment arms, as well as modified capsular tension (Figure 2).[8][12] This is thought to influence the postoperative range of motion by limiting active abduction as well as creating a tendency to inferiorly sublux the humeral head.[27][33] Conversely, thick glenoid components create overstuffing (Figure). Bodrogi et al. recently described a reliable CT-based method to assess changes between pre and post-arthroplasty glenohumeral offset measures.[34] In the absence of humeral head sphericity (particularly in the setting of osteoarthritis), their method relied on the center of the humeral shaft (rather than the center of the humeral head) as described by Jacobsen and Friedman’s line to be independent of retroversion on the glenoid side.[35]
Medullary canal
Finally, the intramedullary canal not only becomes tighter but also increasingly retroverted from proximal to distal.[11] Fixation of the humeral component is widely varied. Diaphyseal press-fit stems induce proximal stress shielding. Cementation is reliable at time zero but difficult in revision. The goals of reduced stress shielding, easier stem revision, and preservation of vascularity have led to a progressive shift towards short metaphyseal stem or stemless fixation.[23] While a comparative cadaveric study revealed decreased micromotion and enhanced rotational stability in cemented stems,[36] optimal stem fixation, length, and filling ratio to avoid stress shielding,[37] subsidence,[38] and misalignment remains controversial.[39]
Glenoid anatomy
Glenoid loosening remains the primary cause of anatomic total shoulder arthroplasty failure.[40] Similar to the humeral side, osteoarthritis appears to modify normal glenoid anatomy significantly. The glenoid seems relatively small and shallow compared to the humerus, with only 9 cm2 of articular surface.[41] The glenoid is pear-shaped with a superior to an inferior dimension of 39 mm an inferior glenoid width averaging 29 mm.[12] There is a radii mismatch between the glenoid and humeral head, while the radius of curvature is greater in the anteroposterior than the superoinferior direction (41 vs. 32 mm).[1] Biomechanically, perfect conformity leads to a more stable joint but increased stress on the glenoid. On the other hand, an increased mismatch in radii will lead to increased translation of the humerus onto the glenoid with rim loading of the glenoid component causing a “rocking horse” effect.[42][43] Based on current techniques, the best compromise appears to be a mismatch ranging between 4 and 8 mm.[44] However, it should be noted that these findings are based on a spherical humeral head. It has been proposed that conformed designs are better suited for elliptical heads.[45]
Glenoid version and inclination
Reported three-dimensional CT-derived measures report mean normal glenoid retroversion of 6 ± 4 degrees and inclination of 7 ± 5 degrees. Retroversion has been correlated (r = 0.7, P < 0.001) to posterior humeral head subluxation (59% ± 7%).[46] The contralateral shoulder may be a reliable model, like side to side differences are limited to 5 degrees in 95% of the cases.[47] It is also important to assess the version in three dimensions, as in cases with >10 degrees version, it is not solely direct posteriorly but also in superior, inferior, and anterior directions.[48] A further important hint when performing anatomic total shoulder arthroplasty is that the version of the inferior part of the glenoid shows substantial less variability compared to the upper part and should therefore be used as the preferred intra-operative landmark in order to achieve adequate implant positioning.[49]
Concerning inclination, Moor et al. proposed the critical shoulder angle as a measure of scapular morphology with the benefit of combining measurements of glenoid inclination and lateral acromion coverage.[50] They identified an angle inferior to 30 degrees as being associated with primary shoulder osteoarthritis. This finding is supported by subsequent biomechanical studies reporting increased joint reaction forces in case of a lower critical shoulder angle.[51][52] Critical shoulder angle >35 degrees is, on the other hand, related to an increased incidence of rotator cuff tears secondary to increased supraspinatus loading to compensate for increased joint instability as a consequence of increased glenohumeral joint shear forces.[50][53][54] In the setting of anatomic total shoulder arthroplasty, an increased critical shoulder angle has been related to an increased incidence of glenoid radiolucencies.[54]
Humeral head subluxation
The Walch classification, with subsequent modifications, is the most common means of assessing glenoid changes secondary to primary osteoarthritis.[55][56] Walch classified glenoid deformity based on posterior glenoid retroversion and humeral head subluxation. In opposition to type A glenoids (symmetrical bone loss), type B glenoids (asymmetrical bone loss) have been associated with progressive posterior glenoid bone loss over time.[57] This factor is important when evaluating posterior humeral head subluxation; in type B3 glenoids, the head might be centered in regard to the glenoid but be posteriorly translated in relation to the scapula. Iannoti et al., by using three-dimensional standardized measures, reported a continuum of measures among the different type B and C glenoids rather than defined categories (B1, B2, B3, and C) in regard to glenoid retroversion and humeral head subluxation.[58] Currently, it is still debated if posterior humeral subluxation is the cause or consequence of increased retroversion.[59] Static posterior humeral head subluxation and posterior glenoid wear have both been associated with premature osteoarthritis in young men and related to higher complication rates after anatomic total shoulder arthroplasty.[60][61][62][63] Recently, Beeler et al. identified a flat acromion roof as a potential risk factor for posterior humeral head subluxation and posterior glenoid wear.[64] This hypothesis was confirmed by a subsequent study by Meyer et al., reporting a median of 4 degrees more glenoid retroversion and a 5 degrees less steep acromion in type B2 and C compared to type A and B1 glenoids (P ≤ 0.022).[65]
Instability
The rotator cuff and the horizontal force couple are critical to glenohumeral stability.[66] By respecting cuff insertion and restoring bony anatomy, force couples should be adequately restored. Soft tissue balancing, by the combination of the anterior subscapularis tendon and capsule release sometimes associated with a capsulorraphy of the redundant posterior capsule, is indicated to reach Matsen’s criteria (40 degrees of external rotation, 60 degrees of internal rotation and a 50% posterior shift of the humeral head over the glenoid).[67] If bony correction is necessary, one should carefully reevaluate adequate humeral implant size as center of rotation likely changed secondary to the additional bone removal. When facing a retroverted glenoid, posterior instability can be compensated for by anteriorly offsetting the humeral head component, leading to a significant anterior humeral displacement on muscle activation as well as an anterior shift of the center of pressure (p<0.05).[68][69] A major downside of this technique, however, is increased tension on the subscapularis with potentially higher rates of subscapularis failures. Chronic irreparable subscapularis deficiency is a contraindication to anatomic total shoulder arthroplasty as it tends to destabilize the joint secondary to an upward migration of the humeral head and eccentric contact pressure onto the glenoid.[70] While subscapularis preserving approaches have been described, most surgeons access the glenohumeral joint by subscapularis detachment with either a tenotomy, peel, or lesser tuberosity osteotomy. Effective subscapularis repair[71] during surgery is therefore mandatory; a review of biomechanical cadaveric studies suggests superior load to failure for the osteotomy at time zero but no difference at cyclic loading[72][73] While de Wilde suggested that a C-block lesser tuberosity osteotomy might prevent postoperative subscapularis fatty infiltration, a recent systematic review reported no statistical difference in clinical and radiological outcomes between tenotomy, peel and osteotomy.[74][75][76] In case of postoperative rupture, a prompt secondary repair can be considered to prevent instability but has been associated with variable results.[77][78] The addition of anterior latissimus dorsi transfer seems biomechanical superior to the pectoralis major transfer in anatomic total shoulder arthroplasty due to an improved internal rotation moment arm and more similar line of pull relative to the subscapularis.[79]
Glenoid bone loss
Correcting glenohumeral bone loss is an important step when implanting the glenoid component. Implanting the component in excessive retroversion will result in posterior translation of the humeral head and subsequent rim-loading known to cause early component loosening.[80][81] According to a finite element model by Farron et al., 10 degrees of retroversion should be considered as the cut-off value.[82] In their analysis, an implant with 20 degrees of retroversion resulted in a 326% increased stress within the cement mantel and a 706% increase of micromotion at the bone-cement interface. Recent work using statistical shape modeling allowed a computer reconstruction of the premorbid glenoid with a precision of about 1 mm and 2 degrees for version and inclination.[83][84] Several techniques to correct retroversion were developed. If version is corrected alone by means of anterior glenoid reaming, it will lead to significant joint line medialization and central cortex perforation when correction exceeds 15 degrees.[85] Consequently, posterior augmented glenoid implants were developed to avoid the medialization of the joint line, with encouraging early results.[86] However, severe deformity has been associated with loosening of such components.[87]
Proper implantation technique avoiding superior inclination or retroversion is thought to be crucial to avoid edge-loading causing micromotion and subsequent breakdown at the bone-implant interface, ultimately leading to aseptic loosening.[82][88] For the same reason, an intact cuff is also mandatory to conserve physiologic joint kinematics and therefore limit polyethylene wear.[89] While most current anatomic total shoulder arthroplasty heads are metallic, experimental studies suggest that a change towards ceramic heads could reduce polyethylene wear rate by up to 26.7%.[90] A wide range of onlay all-polyethylene glenoid shapes (pear-shaped versus elliptic) and sizes are currently available on the market, with no current consensus on optimal designs regarding back-surface (flat versus curved), anchorage (keel versus peg) or level of conformity.[91] Further, a recent cadaveric study comparing inlay (implanted into the bone socket and therefore allowing for circumferential bone support) with onlay components revealed superior outcome regarding joint reaction forces and fatigue failure in favor of the inlay design.[92] There is also renewed interest towards metal-back glenoids in response to the reported encouraging survival rates of modern designs.[93] While the theoretical benefit of more stable fixation and easy conversion to reverse shoulder arthroplasty seems appealing, long-term outcomes are awaited based on the long list of retrieved pre-existing metal-back designs.[94]
Treatment
Clinical Practice Guideline
The goal of this section is to provide clinicians with recommendations based on the best available evidence; to inform clinicians of when there is no evidence; and finally, to help clinicians deliver the best health care possible.
This subsection does not exist. You can ask for it to be created, but consider checking the search results below to see whether the topic is already covered.
Conservative (Nonoperative) Treatment
This subsection does not exist. You can ask for it to be created, but consider checking the search results below to see whether the topic is already covered.
Surgical (Operative) Treatment
Approaches
Deltopectoral
The deltopectoral approach consisted of a 10 to 15 cm skin incision being made from the coracoid process toward the deltoid insertion. The infraclavicular fossa (Mohrenheim fossa) is found, the cephalic vein identified and the consistent medial branches, which give the appearance of the Mercedes Benz symbol, are ligated. A self-retaining retractor is used to maintain exposure between the deltoid and pectoralis major. The subacromial bursa was resected to allow the placement of a Hohmann retractor under the deltoid over the top of the coracoid process. The arm was abducted and internally rotated. The subacromial bursa is resected to allow the placement of a Brown-Deltoid retractor.
Subscapularis Tenotomy
This subsection does not exist. You can ask for it to be created, but consider checking the search results below to see whether the topic is already covered.
Osteotomy of the Lesser Tuberosity
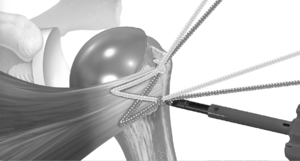
The osteotomy is initiated at the bicipital groove with a 2-mm saw blade and then completed with a curved osteotome. An approximately 2.5 cm2 in the coronal plane and 5 mm thick fleck of lesser tuberosity is taken such that the osteotomy entered the joint medially without violating the humeral head.[95][96]
A complete release of the subscapularis tendon is then performed and the tendon is pushed in the subscapularis fossa. A glenoid retractor is placed anteriorly. The humeral head is resected with a guide or a free-handed anatomic cut respecting native humeral head version and inclination.
Subscapularis Repair
This subsection does not exist. You can ask for it to be created, but consider checking the search results below to see whether the topic is already covered.
Lesser Osteotomy Repair
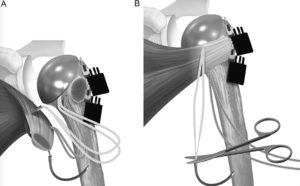
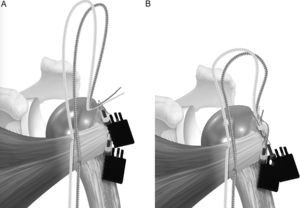
Before placement of the humeral stem, two holes are created with a 2-mm drill bit in the bicipital groove at the superior and inferior aspects of the lesser tuberosity osteotomy. One hole was created in the metaphysis just medial to the lesser tuberosity osteotomy. The sutures are then passed from lateral to medial by entering the bicipital groove, passing around the humeral stem, and exiting medially (Figure 16). A racking hitch is positioned to rest in the bicipital groove. The two sutures are passed through the subscapularis just medial to the lesser tuberosity osteotomy. The needle is removed from each construct to leave two superior and two inferior limbs (Figure 17). Then, one of the superior limbs and one of the inferior limbs were shuttled through the superior racking hitch knot (Figure 18). The suture limbs are passed through a tensioner to remove slack and to tension the repair (Figure 19).
Postoperative Rehabilitation
A sling was worn for 4 weeks following surgery. During the first 4 weeks, these patients only performed active hand, wrist, and elbow exercises, as well as active scapular retraction exercises. At 4 weeks postoperatively, the sling is discontinued and passive forward elevation with a rope and pulley and passive external rotation with a stick were initiated as tolerated. At 8 weeks postoperatively, active assisted progression to active ROM is allowed as tolerated. Strengthening is also routinely started at 8 weeks postoperatively. Activities are allowed as tolerated at 16 weeks postoperatively with a lifetime recommendation for no repetitive lifting over 25 lb (11.3 kg).
Results
This subsection does not exist. You can ask for it to be created, but consider checking the search results below to see whether the topic is already covered.
Complications
This subsection does not exist. You can ask for it to be created, but consider checking the search results below to see whether the topic is already covered.
References
- ↑ 1.0 1.1 McPherson EJ, Friedman RJ, An YH, Chokesi R, Dooley RL. Anthropometric study of normal glenohumeral relationships. J Shoulder Elbow Surg. 1997;6(2):105-12
- ↑ 2.0 2.1 Sharkey NA, Marder RA. The rotator cuff opposes superior translation of the humeral head. Am J Sports Med. 1995;23(3):270-5
- ↑ Lee SB, Kim KJ, O'Driscoll SW, Morrey BF, An KN. Dynamic glenohumeral stability provided by the rotator cuff muscles in the mid-range and end-range of motion. A study in cadavera. J Bone Joint Surg Am. 2000;82(6):849-57
- ↑ Bergmann G, Graichen F, Bender A, Rohlmann A, Halder A, Beier A, et al. In vivo gleno-humeral joint loads during forward flexion and abduction. Journal of biomechanics. 2011;44(8):1543-52
- ↑ 5.0 5.1 Knowles NK, Carroll MJ, Keener JD, Ferreira LM, Athwal GS. A comparison of normal and osteoarthritic humeral head size and morphology. J Shoulder Elbow Surg. 2016;25(3):502-9
- ↑ 6.0 6.1 6.2 Alolabi B, Youderian AR, Napolitano L, Szerlip BW, Evans PJ, Nowinski RJ, et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1740-6
- ↑ Buchler P, Farron A. Benefits of an anatomical reconstruction of the humeral head during shoulder arthroplasty: a finite element analysis. Clin Biomech (Bristol, Avon). 2004;19(1):16-23
- ↑ 8.0 8.1 Boileau P, Walch G. The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br. 1997;79(5):857-65
- ↑ Jun BJ, Iannotti JP, McGarry MH, Yoo JC, Quigley RJ, Lee TQ. The effects of prosthetic humeral head shape on glenohumeral joint kinematics: a comparison of non-spherical and spherical prosthetic heads to the native humeral head. J Shoulder Elbow Surg. 2013;22(10):1423-32
- ↑ Berghs BM, Derveaux T, Speeckaert W, Vanslambrouck K, De Wilde LF. Three-dimensional analysis of the orientation and the inclination of the rotator cuff footprint. J Shoulder Elbow Surg. 2011;20(4):637-45
- ↑ 11.0 11.1 Robertson DD, Yuan J, Bigliani LU, Flatow EL, Yamaguchi K. Three-dimensional analysis of the proximal part of the humerus: relevance to arthroplasty. J Bone Joint Surg Am. 2000;82(11):1594-602
- ↑ 12.0 12.1 12.2 12.3 12.4 Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500
- ↑ Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-8
- ↑ Getz CL, Ricchetti ET, Verborgt O, Brolin TJ. Normal and Pathoanatomy of the Arthritic Shoulder: Considerations for Shoulder Arthroplasty. J Am Acad Orthop Surg. 2019;27(24):e1068-e76
- ↑ Barth J, Garret J, Boutsiadis A, Sautier E, Geais L, Bothorel H, et al. Is global humeral head offset related to intramedullary canal width? A computer tomography morphometric study. J Exp Orthop. 2018;5(1):35
- ↑ Muench, L.N., Otto, A., Kia, C. et al. Rotational range of motion of elliptical and spherical heads in shoulder arthroplasty: a dynamic biomechanical evaluation. Arch Orthop Trauma Surg 2020 https://doi.org/10.1007/s00402-020-03587-0
- ↑ Terrier A, Ramondetti S, Merlini F, Pioletti DD, Farron A. Biomechanical consequences of humeral component malpositioning after anatomical total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(8):1184-90
- ↑ Youderian AR, Ricchetti ET, Drews M, Iannotti JP. Determination of humeral head size in anatomic shoulder replacement for glenohumeral osteoarthritis. J Shoulder Elbow Surg. 2014;23(7):955-63
- ↑ Favre P, Moor B, Snedeker JG, Gerber C. Influence of component positioning on impingement in conventional total shoulder arthroplasty. Clin Biomech (Bristol, Avon). 2008;23(2):175-83
- ↑ Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am. 2004;86(3):575-80
- ↑ Vaesel MT, Olsen BS, Sojbjerg JO, Helmig P, Sneppen O. Humeral head size in shoulder arthroplasty: a kinematic study. J Shoulder Elbow Surg. 1997;6(6):549-55
- ↑ Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-6
- ↑ 23.0 23.1 Keener JD, Chalmers PN, Yamaguchi K. The Humeral Implant in Shoulder Arthroplasty. J Am Acad Orthop Surg. 2017;25(6):427-38
- ↑ Hammond G, Tibone JE, McGarry MH, Jun BJ, Lee TQ. Biomechanical comparison of anatomic humeral head resurfacing and hemiarthroplasty in functional glenohumeral positions. J Bone Joint Surg Am. 2012;94(1):68-76
- ↑ Jeong J, Bryan J, Iannotti JP. Effect of a variable prosthetic neck-shaft angle and the surgical technique on replication of normal humeral anatomy. J Bone Joint Surg Am. 2009;91(8):1932-41
- ↑ Bryce CD, Davison AC, Okita N, Lewis GS, Sharkey NA, Armstrong AD. A biomechanical study of posterior glenoid bone loss and humeral head translation. J Shoulder Elbow Surg. 2010;19(7):994-1002
- ↑ 27.0 27.1 Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492-7
- ↑ Ovesen J, Nielsen S. Prosthesis position in shoulder arthroplasty. A cadaver study of the humeral component. Acta Orthop Scand. 1985;56(4):330-1
- ↑ Pearl ML, Volk AG. Retroversion of the proximal humerus in relationship to prosthetic replacement arthroplasty. J Shoulder Elbow Surg. 1995;4(4):286-9
- ↑ Doyle AJ, Burks RT. Comparison of humeral head retroversion with the humeral axis/biceps groove relationship: a study in live subjects and cadavers. J Shoulder Elbow Surg. 1998;7(5):453-7
- ↑ Itamura J, Dietrick T, Roidis N, Shean C, Chen F, Tibone J. Analysis of the bicipital groove as a landmark for humeral head replacement. J Shoulder Elbow Surg. 2002;11(4):322-6
- ↑ Raniga S, Knowles NK, West E, Ferreira LM, Athwal GS. The Walch type B humerus: glenoid retroversion is associated with torsional differences in the humerus. J Shoulder Elbow Surg. 2019;28(9):1801-8
- ↑ Hsu HC, Wu JJ, Chen TH, Lo WH, Yang DJ. The influence of abductor lever-arm changes after shoulder arthroplasty. J Shoulder Elbow Surg. 1993;2(3):134-40
- ↑ Bodrogi A, Athwal GS, Howard L, Zhang T, Lapner P. A reliable method of determining glenohumeral offset in anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28(8):1609-16
- ↑ Jacobson SR, Mallon WJ. The glenohumeral offset ratio: A radiographic study. J Shoulder Elbow Surg. 1993;2(3):141-6
- ↑ Harris TE, Jobe CM, Dai QG. Fixation of proximal humeral prostheses and rotational micromotion. J Shoulder Elbow Surg. 2000;9(3):205-10
- ↑ Raiss P, Schnetzke M, Wittmann T, Kilian CM, Edwards TB, Denard PJ, et al. Postoperative radiographic findings of an uncemented convertible short stem for anatomic and reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28(4):715-23
- ↑ Tross AK, Lädermann A, Wittmann T, Schnetzke M, Nolte PC, Collin P, et al. Subsidence of Uncemented Short Stems in Reverse Shoulder Arthroplasty-A Multicenter Study. J Clin Med. 2020;9(10)
- ↑ Lädermann A, Chiu JC, Cunningham G, Herve A, Piotton S, Bothorel H, et al. Do short stems influence the cervico-diaphyseal angle and the medullary filling after reverse shoulder arthroplasties? Orthop Traumatol Surg Res. 2020;106(2):241-6
- ↑ Terrier A, Goetti P, Becce F, Farron A. Reduction of scapulohumeral subluxation with posterior augmented glenoid implants in anatomic total shoulder arthroplasty: Short-term 3D comparison between pre- and post-operative CT. Orthop Traumatol Surg Res. 2020;106(4):681-6
- ↑ Kwon YW, Powell KA, Yum JK, Brems JJ, Iannotti JP. Use of three-dimensional computed tomography for the analysis of the glenoid anatomy. J Shoulder Elbow Surg. 2005;14(1):85-90
- ↑ Anglin C, Wyss UP, Pichora DR. Shoulder prosthesis subluxation: theory and experiment. J Shoulder Elbow Surg. 2000;9(2):104-14
- ↑ Franklin JL, Barrett WP, Jackins SE, Matsen FA, 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46
- ↑ Schoch B, Abboud J, Namdari S, Lazarus M. Glenohumeral Mismatch in Anatomic Total Shoulder Arthroplasty. JBJS Rev. 2017;5(9):e1
- ↑ Jun BJ, Lee TQ, McGarry MH, Quigley RJ, Shin SJ, Iannotti JP. The effects of prosthetic humeral head shape on glenohumeral joint kinematics during humeral axial rotation in total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25(7):1084-93
- ↑ Gauci MO, Deransart P, Chaoui J, Urvoy M, Athwal GS, Sanchez-Sotelo J, et al. Three-dimensional geometry of the normal shoulder: a software analysis. J Shoulder Elbow Surg. 2020;29(12):e468-e477
- ↑ Verhaegen F, Plessers K, Verborgt O, Scheys L, Debeer P. Can the contralateral scapula be used as a reliable template to reconstruct the eroded scapula during shoulder arthroplasty? J Shoulder Elbow Surg. 2018;27(6):1133-8
- ↑ Terrier A, Ston J, Larrea X, Farron A. Measurements of three-dimensional glenoid erosion when planning the prosthetic replacement of osteoarthritic shoulders. Bone Joint J. 2014;96-B(4):513-8
- ↑ De Wilde LF, Verstraeten T, Speeckaert W, Karelse A. Reliability of the glenoid plane. J Shoulder Elbow Surg. 2010;19(3):414-22
- ↑ 50.0 50.1 Moor BK, Bouaicha S, Rothenfluh DA, Sukthankar A, Gerber C. Is there an association between the individual anatomy of the scapula and the development of rotator cuff tears or osteoarthritis of the glenohumeral joint?: A radiological study of the critical shoulder angle. Bone Joint J. 2013;95-B(7):935-41
- ↑ Engelhardt C, Farron A, Becce F, Place N, Pioletti DP, Terrier A. Effects of glenoid inclination and acromion index on humeral head translation and glenoid articular cartilage strain. J Shoulder Elbow Surg. 2017;26(1):157-64
- ↑ Viehofer AF, Snedeker JG, Baumgartner D, Gerber C. Glenohumeral joint reaction forces increase with critical shoulder angles representative of osteoarthritis-A biomechanical analysis. J Orthop Res. 2016;34(6):1047-52
- ↑ Gerber C, Snedeker JG, Baumgartner D, Viehofer AF. Supraspinatus tendon load during abduction is dependent on the size of the critical shoulder angle: A biomechanical analysis. J Orthop Res. 2014;32(7):952-7
- ↑ 54.0 54.1 Watling JP, Sanchez JE, Heilbroner SP, Levine WN, Bigliani LU, Jobin CM. Glenoid component loosening associated with increased critical shoulder angle at midterm follow-up. J Shoulder Elbow Surg. 2018;27(3):449-54
- ↑ Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-60
- ↑ Bercik MJ, Kruse K, 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-6
- ↑ Walker KE, Simcock XC, Jun BJ, Iannotti JP, Ricchetti ET. Progression of Glenoid Morphology in Glenohumeral Osteoarthritis. J Bone Joint Surg Am. 2018;100(1):49-56
- ↑ Iannotti JP, Jun BJ, Patterson TE, Ricchetti ET. Quantitative Measurement of Osseous Pathology in Advanced Glenohumeral Osteoarthritis. J Bone Joint Surg Am. 2017;99(17):1460-8
- ↑ Domos P, Checchia CS, Walch G. Walch B0 glenoid: pre-osteoarthritic posterior subluxation of the humeral head. J Shoulder Elbow Surg. 2018;27(1):181-8
- ↑ Walch G, Ascani C, Boulahia A, Nove-Josserand L, Edwards TB. Static posterior subluxation of the humeral head: an unrecognized entity responsible for glenohumeral osteoarthritis in the young adult. J Shoulder Elbow Surg. 2002;11(4):309-14
- ↑ Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg. 2012;21(11):1526-33
- ↑ Sabesan VJ, Callanan M, Youderian A, Iannotti JP. 3D CT assessment of the relationship between humeral head alignment and glenoid retroversion in glenohumeral osteoarthritis. J Bone Joint Surg Am. 2014;96(8):e64
- ↑ Jacxsens M, Van Tongel A, Henninger HB, De Coninck B, Mueller AM, De Wilde L. A three-dimensional comparative study on the scapulohumeral relationship in normal and osteoarthritic shoulders. J Shoulder Elbow Surg. 2016;25(10):1607-15
- ↑ Beeler S, Hasler A, Gotschi T, Meyer DC, Gerber C. Different acromial roof morphology in concentric and eccentric osteoarthritis of the shoulder: a multiplane reconstruction analysis of 105 shoulder computed tomography scans. J Shoulder Elbow Surg. 2018;27(12):e357-e66
- ↑ Meyer DC, Riedo S, Eckers F, Carpeggiani G, Jentzsch T, Gerber C. Small anteroposterior inclination of the acromion is a predictor for posterior glenohumeral erosion (B2 or C). J Shoulder Elbow Surg. 2019;28(1):22-7
- ↑ Goetti P, Denard PJ, Collin P, Ibrahim M, Hoffmeyer P, Lädermann A. Shoulder biomechanics in normal and selected pathological conditions. EFORT Open Reviews. 2020;5(8):508-18
- ↑ Matsen F, Lippitt S. Shoulder surgery: principles and procedures. Matsen F, Lippitt S, editors. Philadelphia:: Saunders; 2003
- ↑ Lewis GS, Conaway WK, Wee H, Kim HM. Effects of anterior offsetting of humeral head component in posteriorly unstable total shoulder arthroplasty: Finite element modeling of cadaver specimens. J Biomech. 2017;53:78-83
- ↑ Kim HM, Chacon AC, Andrews SH, Roush EP, Cho E, Conaway WK, et al. Biomechanical benefits of anterior offsetting of humeral head component in posteriorly unstable total shoulder arthroplasty: A cadaveric study. J Orthop Res. 2016;34(4):666-74
- ↑ Terrier A, Larrea X, Malfroy Camine V, Pioletti DP, Farron A. Importance of the subscapularis muscle after total shoulder arthroplasty. Clin Biomech (Bristol, Avon). 2013;28(2):146-50
- ↑ Denard PJ, Noyes MP, Lädermann A. A Tensionable Method for Subscapularis Repair after Shoulder Arthroplasty. JSES Open Access. 2018;2(4):205-10
- ↑ Virk MS, Aiyash SS, Frank RM, Mellano CS, Shewman EF, Wang VM, Romeo AA. Biomechanical comparison of subscapularis peel and lesser tuberosity osteotomy for double-row subscapularis repair technique in a cadaveric arthroplasty model. J Orthop Surg Res. 2019;14(1):391
- ↑ Van Thiel GS, Wang VM, Wang FC, Nho SJ, Piasecki DP, Bach BR, Jr., Romeo AA. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(5):657-63
- ↑ Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Healing rates and subscapularis fatty infiltration after lesser tuberosity osteotomy versus subscapularis peel for exposure during shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(3):396-402
- ↑ Choate WS, Kwapisz A, Momaya AM, Hawkins RJ, Tokish JM. Outcomes for subscapularis management techniques in shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2018;27(2):363-70
- ↑ De Wilde LF, De Coninck T, De Neve F, Berghs BM. Subscapularis release in shoulder replacement determines structural muscular changes. Clin Orthop Relat Res. 2012;470(8):2193-201
- ↑ Miller BS, Joseph TA, Noonan TJ, Horan MP, Hawkins RJ. Rupture of the subscapularis tendon after shoulder arthroplasty: diagnosis, treatment, and outcome. J Shoulder Elbow Surg. 2005;14(5):492-6
- ↑ Shi LL, Jiang JJ, Ek ET, Higgins LD. Failure of the lesser tuberosity osteotomy after total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(2):203-9
- ↑ Werthel JD, Schoch BS, Hooke A, Sperling JW, An KN, Valenti P, Elhassan B. Biomechanical Effectiveness of Tendon Transfers to Restore Active Internal Rotation in Shoulder with Deficient Subscapularis with and without Reverse Shoulder Arthroplasty. J Shoulder Elbow Surg. 2021 May;30(5):1196-1206
- ↑ Nyffeler RW, Sheikh R, Atkinson TS, Jacob HA, Favre P, Gerber C. Effects of glenoid component version on humeral head displacement and joint reaction forces: an experimental study. J Shoulder Elbow Surg. 2006;15(5):625-9
- ↑ Shapiro TA, McGarry MH, Gupta R, Lee YS, Lee TQ. Biomechanical effects of glenoid retroversion in total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 Suppl):S90-5
- ↑ 82.0 82.1 Farron A, Terrier A, Buchler P. Risks of loosening of a prosthetic glenoid implanted in retroversion. J Shoulder Elbow Surg. 2006;15(4):521-6
- ↑ Abler D, Berger S, Terrier A, Becce F, Farron A, Buchler P. A statistical shape model to predict the premorbid glenoid cavity. J Shoulder Elbow Surg. 2018;27(10):1800-8
- ↑ Plessers K, Vanden Berghe P, Van Dijck C, Wirix-Speetjens R, Debeer P, Jonkers I, Vander Sloten J. Virtual reconstruction of glenoid bone defects using a statistical shape model. J Shoulder Elbow Surg. 2018;27(1):160-6
- ↑ Clavert P, Millett PJ, Warner JJ. Glenoid resurfacing: what are the limits to asymmetric reaming for posterior erosion? J Shoulder Elbow Surg. 2007;16(6):843-8
- ↑ Ghoraishian M, Abboud JA, Romeo AA, Williams GR, Namdari S. Augmented glenoid implants in anatomic total shoulder arthroplasty: review of available implants and current literature. J Shoulder Elbow Surg. 2019;28(2):387-95
- ↑ Ho JC, Amini MH, Entezari V, Jun BJ, Alolabi B, Ricchetti ET, Iannoti JP. Clinical and Radiographic Outcomes of a Posteriorly Augmented Glenoid Component in Anatomic Total Shoulder Arthroplasty for Primary Osteoarthritis with Posterior Glenoid Bone Loss. J Bone Joint Surg Am. 2018;100(22):1934-48
- ↑ Karelse A, Van Tongel A, Verstraeten T, Poncet D, De Wilde LF. Rocking-horse phenomenon of the glenoid component: the importance of inclination. J Shoulder Elbow Surg. 2015;24(7):1142-8
- ↑ Braun S, Schroeder S, Mueller U, Sonntag R, Buelhoff M, Kretzer JP. Influence of joint kinematics on polyethylene wear in anatomic shoulder joint arthroplasty. J Shoulder Elbow Surg. 2018;27(9):1679-85
- ↑ Mueller U, Braun S, Schroeder S, Schroeder M, Sonntag R, Jaeger S, Kretzer JP. Influence of humeral head material on wear performance in anatomic shoulder joint arthroplasty. J Shoulder Elbow Surg. 2017;26(10):1756-64
- ↑ Junaid S, Sanghavi S, Anglin C, Bull A, Emery R, Amis AA, Hansen Ul. Treatment of the Fixation Surface Improves Glenoid Prosthesis Longevity in vitro. Journal of biomechanics. 2017;61:81-7
- ↑ Gagliano JR, Helms SM, Colbath GP, Przestrzelski BT, Hawkins RJ, DesJardins JD. A comparison of onlay versus inlay glenoid component loosening in total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(7):1113-20
- ↑ Castagna A, Randelli M, Garofalo R, Maradei L, Giardella A, Borroni M. Mid-term results of a metal-backed glenoid component in total shoulder replacement. J Bone Joint Surg Br. 2010;92(10):1410-5
- ↑ Castagna A, Garofalo R. Journey of the glenoid in anatomic total shoulder replacement. Shoulder Elbow. 2019;11(2):140-8
- ↑ Giuseffi SA, Wongtriratanachai P, Omae H, Cil A, Zobitz ME, An KN, Sperling JW, Steinmann SP. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg 2012;21:1087-95.
- ↑ Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am 2005;87(Suppl 2):1-8