Shoulder:Biomechanics
From Goetti et al.,[1][2] with permission.
Contents
Bullet Points
- The stability of the glenohumeral joint depends on soft tissue stabilizers, bone morphology and dynamic stabilizers such as the rotator cuff and long head of the biceps tendon.
- Shoulder stabilization techniques include anatomic procedures such as repair of the labrum or restoration of bone loss, but also non-anatomic options such as remplissage or tendon transfers.
- Rotator cuff repair should restore the cuff anatomy, reattach the rotator cable and respect the coracoacromial arch whenever possible. Tendon transfer, superior capsular reconstruction or balloon implantation have been proposed for irreparable lesions.
- Shoulder rehabilitation should focus on restoring balanced glenohumeral and scapular force couples in order to avoid an upward migration of the humeral head and secondary cuff impingement. The primary goal of cuff repair is to be as anatomic as possible and to create a biomechanically favourable environment for tendon healing.
- The biomechanics of the shoulder relies on careful balancing between stability and mobility. A thorough understanding of normal and degenerative shoulder anatomy is necessary as the goal of anatomic total shoulder arthroplasty is to reproduce premorbid shoulder kinematics.
- With reported joint reaction forces up to 2.4 times bodyweight, failure to restore anatomy and therefore provide a stable fulcrum will result in early implant failure secondary to glenoid loosening.
- The high variability of proximal humeral anatomy can be addressed with modular stems or stemless humeral components. The development of three-dimensional planning has led to a better understanding of the complex nature of glenoid bone deformity in eccentric osteoarthritis.
- The treatment of cuff tear arthropathy patients was revolutionized by the arrival of Grammont’s reverse shoulder arthroplasty. The initial design medialized the center of rotation and distalized the humerus, allowing up to a 42% increase in the deltoid moment arm.
- More modern reverse designs have maintained the element of restored stability but sought a more anatomic postoperative position to minimize complications and maximize rotational range of motion.
Keywords
Anatomy; glenohumeral instability; humerus; ligaments; rehabilitation; rotator cuff; scapula; therapeutic implications; shoulder pathology; glenohumeral arthritis; replacement; prosthesis design; complication; humeral and glenoid morphology; polyethylene; mismatch; neck shaft angle; inclination; onlay; inlay; distalization; glenosphere size; excentricity.
Introduction
The biomechanics of the shoulder are highly complex. First, it is composed of four joints (glenohumeral, acromioclavicular, scapulothoracic, and sternoclavicular). The glenohumeral joint has six degrees of freedom and is the most mobile joint in the human body, allowing the hand to reach a wide range of positions. This mobility can be further enhanced by translation of the humeral head on the glenoid, but the consequence of this tremendous mobility is perhaps a predisposition to instability and impingements. Second, mobility is assumed by 18 muscles that act in synergy. Consequently, decoupling/isolating them is impossible, making precise kinematic analysis and clinical examination difficult. Third, the glenohumeral joint has the characteristics of an active non-weight-bearing joint, leading to major bony and muscular modifications and frequent tendon overuse.
When looking at the shoulder as a functional unit, it appears that several factors need consideration. To function normally, the shoulder needs all the anatomic structures to work in a chain. Form will allow function.[3] First, the central nervous system provides a signal to the muscletendon unit. By contracting, the muscle transmits its tension to the tendon, which then acts as a lever arm on the joint. To be efficient, such a system requires a stable fulcrum. The necessary stability is provided by static and dynamic factors such as bony contours, ligaments, labrum, capsule, etc.
The specificity of biomechanically relevant parameters, such as, for example, joint reaction forces, is that they cannot be measured in vivo without invasive procedures.[4] Our knowledge therefore mainly relies on experimental cadaveric studies[5] or computational modelling.[6] These simulations have become more sophisticated in recent years, allowing the inclusion of an increasing number of variables with the ability to adjust both pathology and patient-specific characteristics.[7] This ongoing process will without doubt call into question prior assumptions and allow further insights into shoulder biomechanics. It is crucial to understand the basic principles of shoulder biomechanics and their modifications in the most common pathologies encountered in daily practice.
Acromioclavicular Joint
The acromioclavicular joint is stabilized both by static and dynamic stabilizers. The static stabilizers include 1) the four acromioclavicular ligaments (superior, inferior, anterior, and posterior), 2) the lateral coracoclavicular ligaments (conoid and trapezoid), 3) the medial coracoclavicular ligaments (Figure and Video) and 4) the coracoacromial ligament.[8][9] The latter, when transferred during standard Weaver-Dunn repair is only 1/4 as strong as the intact coracoclavicular ligaments; such technique of stabilization does not provide sufficient strength and is considered by many as obsolete.[10][11][12]
 Medial coracoclavicular ligament (asterisk) in a right shoulder region. View from in front. C clavicle, CP coracoid process (horizontal portion), DM deltoid muscle (resected), PM pectoralis minor, SM subclavius muscle. Reprinted from Stimec et al.,[8] with permission. |
The capsular ligaments acted as a primary restraint to posterior displacement of the clavicle (Video).[13]
The superior ligament is the strongest, followed by posterior. Both ligaments provide the most restraint to posterior translation of the acromioclavicular joint and must be preserved during a Mumford procedure. The coracoclavicular ligaments (trapezoid and conoid) provides vertical stability. The dynamic stabilizers include the deltoid and trapezius muscles.[14]
The coracoclavicular ligaments’ main contribution is to vertical stability. However, its double bundle configuration contributes also partially to horizontal stability due to their relative orientation.[15][16]
After lesion of the acromioclavicular ligaments, the conoid ligament acts as the primary restraint against anterior and superior loading, while the trapezoid functioned as the primary restraint against posterior loading.[17] When a load is applied in a superior direction, the conoid ligament fails first in its midsubstance region.[18] [19]
During elevation of the arm, the clavicle with respect to the thorax generally undergoes elevation (11 to 15 degrees), retraction (15 to 29 degrees), and posterior long-axis rotation (15 to 31 degrees). Motion of the scapula (protraction-retraction) plays a major role in the motion at the acromioclavicular joint.[20]
Instability
Static stabilizers
Static stability of the glenohumeral joint is provided by the capsulolabral structures as well as the bony anatomy of the glenoid. Historically, significant effort was placed on understanding the importance of the anterior capsulolabral structures, due to the fact that these structures are classically torn in the case of anterior shoulder instability.[21] The glenohumeral ligaments are a thickening of the joint capsule and represent the primary static stabilizers. To allow a high degree of shoulder mobility they only become tight at the end-ranges of motion. The superior glenohumeral ligament is tight in adduction, the middle at 45 degrees of abduction and the inferior glenohumeral when the shoulder is brought to 90 degrees of abduction in external rotation.[22] The inferior glenohumeral ligament is therefore considered the strongest and most important soft tissue stabilizer. Structurally it can be avulsed from the glenoid side resulting in an antero-inferior labral lesion, as well as from the humeral side resulting in the less-frequent humeral avulsion of the glenohumeral ligament (HAGL) lesion.[23][24] The postero-inferior capsule and posterior inferior glenohumeral ligament are not as robust as their anterior counterparts,[25] but it is often felt to be necessary to ‘balance’ both inferior ligaments during a soft tissue repair for instability. Laxity is a normal, physiologic and asymptomatic finding, that center of rotationresponds to translation of the humeral head in any direction to the glenoid.[26] Hyperlaxity is constitutional, multidirectional, bilateral and asymptomatic. Hyperlaxity of the shoulder is probably best defined as external rotation with the elbow at the side equal to or greater than 85 degrees.[27] This nonpathological finding is a risk factor for instability but does not by itself demand treatment unless there is clear pathological laxity. Pathological laxity of the inferior glenohumeral ligament is observed when passive abduction in neutral rotation in the glenohumeral joint is above 105 degrees, there is apprehension above 90 degrees of abduction, or if a difference of more than 20 degrees between the two shoulders is noted.[28][29] Pathological laxity is often multidirectional and associated with a redundant capsule leading to an increased glenohumeral volume.[30] Biomechanical studies have focused on evaluating the effectiveness of soft tissue procedures to reduce capsular volume. Cadaveric models created by stretching the capsule 10–30% beyond the maximal range of motion, revealed that 1 cm capsular shifts were effective to reduce capsular volume by an average 33.7% (range, 25.3% to 44.6%).[31][32][33] Ponce et al. further reported a linear relationship between the number of 1 cm stitches and capsular volume, each plication reducing the volume by approximately 10%.[34] Lastly, while both capsular plication and rotator interval closure have been reported to be effective in restoring intact range of motion after capsular stretching, the addition of an interval closure has the benefit of better restoring humeral head translation at 60 degrees of abduction.[33][35]
The osseous glenoid is relatively flat, the biomechanical role of the glenoid cartilage and labrum is to double the depth of the glenoid socket and therefore enhance the contact area with the humeral head.[36][37][38] This is further believed to stabilize the joint by helping to centre the humeral head when compressed against the glenoid by the rotator cuff muscles (concavity compression mechanism). A complete loss of the anterior labrum has been reported to decrease the contact area by 7% to 15%, and increase the mean contact pressure by 8% to 20%.[8] A biomechanical study by Hara et al. identified the anteroinferior labrum as being the weakest point, with a mean force necessary to cause a rupture of 3.84 ± 1.00 kg/5 mm.[39] Finally, it was postulated that an intact labrum could help create a negative intra-articular pressure (vacuum effect); this effect is, however, thought to be marginal when the rotator cuff muscles are contracted.[40][41][42] Despite these important stabilizing effects, Itoi et al revealed that soft tissues alone play only a minor role in glenohumeral stability in mid-range of motion.[43]
Glenoid bone defects and morphology
An important concept regarding glenohumeral joint stability is the concavity compression principle, which centres the humeral head on the glenoid. This centring mechanism is the result of the rotator cuff compressing the humeral head against the glenoid cavity, and is one reason why an anterior glenoid rim defect predisposes to recurrent anterior instability.[44] While there is some controversy, 15% to 20% glenoid bone loss seems to be the cutoff value for soft tissue repair.[45][46] Shin et al. demonstrated that in case of an anterior defect ≥ 15%, a soft tissue procedure (Bankart) is unable to restore normal shoulder kinematics and even leads to postero-inferior translation of the humeral head in abduction and external rotation.[46] On the other hand, bone grafting (glenoidplasty) can successfully reconstruct glenoid curvature and depth and therefore restore stability.[44][47] Another key point is the reduced contact area and increased articular contact pressure induced by bony glenoid defects.[8] While iliac bone graft (Eden-Hybinette), articular distal clavicle autografts and coracoid transfer (Latarjet or Bristow) can all restore normal values, the correct position and orientation of the bone graft is important.[48][49] The Latarjet will, however, be limited by the amount of bone that can be harvested.
Young et al. reported mean values of 26.4 ± 2.9 mm and 9.3 ± 1.4 mm for length and thickness respectively.[50] A graft placed in too lateral of a position will lead to an increased anterior-inferior peak contact pressure, whereas a recessed graft will lead to high edge loading. To avoid increased inferior contact pressure, the current evidence suggests orientating the coracoid bone graft in an inferior direction.[51] The congruent-arc modification of the original Latarjet technique further allows the reconstruction of larger defects by matching the shape of the graft to that of the glenoid.[52] The use of a distal tibial osteochondral allograft respects all these biomechanical principles and has been shown to be a valid alternative in the absence of reliable autograft.[53]
During posterior shoulder dislocation, reverse Bankart lesions are only present in isolation in 51% of cases.[54] They are, however, sufficient to increase posterior translation and inferior translation of the humerus in the sulcus position by 86% and 31% respectively.[55] Additionally, glenoid retroversion is more common in posterior instability and appears to predispose to posterior instability.[56] Every five-degree increment of retroversion led to an additional posterior decentralization of the humeral head overall by (average ± standard deviation) 2.0 mm ± 0.3 in the intact and 2.0 mm ± 0.7 in the detached labrum condition. Bony alignment in terms of glenoid retroversion angle plays an important role in joint centration and posterior translation, especially in retroversion angles greater than 10 degrees.[57] Labral injury from repetitive posterior loading or instability can range from a posterior labral tear to an incomplete, concealed avulsion of the postero-inferior labrum (also known as ‘Kim lesion’) to a reverse Bankart lesion. Glenoid retroversion beyond the average five degrees to 10 degrees has been shown to be a risk factor for developing subsequent posterior instability in a prospective study of healthy subjects. For every one degree increase in glenoid retroversion, the risk for posterior instability increase by 17%.[58]
Humeral bone defects
A Malgaigne lesion[59] also called a Hill–Sachs lesion[60] describes the grooved defect of the humeral head. This frequently unrecognized complication of anterior dislocation of the shoulder joint is the result of compression of the posterolateral head upon the anterior glenoid rim. The presence of humeral bone loss has been linked with recurrent instability after open or arthroscopic shoulder stabilization.[61][62] Cadaveric studies have revealed that humeral bone defects as small as 12.5% of the humeral head diameter will affect joint stability, which can be restored with allograft reconstruction. However, an isolated 25% bone loss was not shown to be sufficient to explain recurrent instability on its own.[63][64][65] In other words, glenoid bone loss is required as well. Clinically, the more common alternative to allograft reconstruction is the remplissage procedure, which insets the posterior capsule and infraspinatus tendon into the lesion. This procedure medializes the insertion of the posterior structures to prevent engagement and also decreases anterior translation of the humeral head. A review identified 10 biomechanical studies of which only one reported persistent engagement after a remplissage procedure in the presence of a 25% humeral head defect.[66] The same study further compared the remplissage to the Latarjet and found that 84% of specimens (27 of 32 testing scenarios) stabilized after remplissage, and 94% of specimens (30 of 32 testing scenarios) stabilized after the Latarjet procedure. This was, however, not statistically significant and the authors concluded that both techniques are effective.[67] Nevertheless, at maximum external rotation at 60 degrees of abduction, remplissage altered the kinematics of the glenohumeral joint by shifting posteriorly and inferiorly the apex of the humeral head.[68] Moreover, while often described as an inset of the infraspinatus tendon, the procedure is in fact a capsulomyodesis of the infraspinatus and teres minor;[69] this has not only been proven in anatomic investigation, but also follows normal form as the tendon does not extend very far medially from its normal insertion.
For posterior instability, the McLaughlin procedure[70] using the detached subscapularis tendon has been described for locked posterior instability in presence of a reverse Malgaigne (Hill–Sachs) lesion. This technique has been subsequently modified as either a reverse remplissage[71] or an osteotomy of the lesser tuberosity with the attached subscapularis (Hughes and Neer method) to allow additional bone support to articular cartilage with satisfactory outcome both in acute and chronic setting.[72][73]
Bipolar defects
Neither glenoid nor humeral head bone loss can be viewed individually. Just as they occur together at the time of injury, they interact in the risk of recurrent instability. The concept of the glenoid track has emerged as a way to understand this relationship. The concept was first proposed by Yamamoto et al., who used three-dimensional computed tomography (CT) scans to reveal that the normal glenoid track is 84% ± 14% of the glenoid width.[74] Subsequently this was validated in live subjects where the value was determined to be 83%. This concept is in fact the continuation of the work by Burkhart and De Beer on engaging vs. non-engaging Hill–Sachs lesions.[75] Di Giacomo et al. further refined this to the on-track and off-track concept, stating that glenoid bone loss will result in a reduction in the width of the glenoid track.[76] In the setting of glenoid bone loss, the glenoid track decreases. The glenoid track in the bone loss situation is determined by subtracting the width of the defect from 83% of the original glenoid width, which is thought to be the width in the absence of a glenoid.[77] Then, the width of the Hill–Sachs defect from the origin of the infraspinatus to the most medial extent of the defect is measured and compared to the glenoid track to determine whether it exceeds the glenoid track (‘off-track’) or is less than the glenoid track (‘on-track’).
Dynamic stabilization (rotator cuff, conjoint tendon and long head of the biceps)
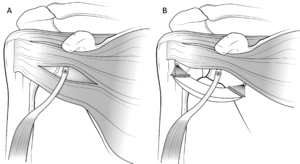
Dynamic stability of the glenohumeral joint is provided by the muscular structures during the mid-points of range of motion. As stated above, the rotator cuff is key to the concavity-compression concept in which it actively contributes to stability in opposition to the deltoid and pectoralis muscles (which tend to destabilize the joint superiorly and anteriorly).[78][79] The cuff contributes to anterior (external rotators) and posterior (internal rotators) stability in cadaveric[80][81] and electromyographic studies.[82] While all rotator cuff muscles contribute to anterior joint stability, the subscapularis seems to be the least effective at endrange of motion in opposition to the long head of the biceps.[83] In addition to the previously mentioned bony augmentation, the Latarjet procedure and its variant the Bristow combine (1) the ligamentous effect by augmentation of the coracoacromial ligament by the inferior glenohumeral ligament, (2) a muscular effect (hammock effect) by lowering the inferior part of the subscapularis, which is mainly efficient in mid-range motion (Figure A and B),[84] as well as (3) a sling effect induced by the conjoint tendon forming an anterior rampart especially efficacious in endrange motion (Figure). The two latter effects have often been confused in the literature.
According to a cadaveric study by Yamamoto et al., the hammock and sling effects appear to be the primary stabilizers and account for 51% to 62% of shoulder stability in mid-range of motion, and up to 76% to 77% at 90 degrees of abduction and maximal external rotation (end-range motion).[85] The Latarjet technique further leads to an enhanced sling effect in comparison to the Bristow procedure due to the inferior graft position and subsequent conjoint tendon orientation and trajectory (Figure 2).[86]
These hammock and sling effects are also the central point of the recently developed dynamic anterior stabilization (DAS) procedure. In this technique the long head of the biceps, in place of the conjoint tendon, it transferred through a subscapularis split to the anterior glenoid margin.[87] The DAS results in decreased anterior glenohumeral translation depending on the glenoid defect conditions. As compared with isolated Bankart repair, DAS shows significantly less relative anterior translation in 10% glenoid defects at translation forces of 20 N (0.3 ± 1.7 mm vs. 2.2 ± 1.8 mm, P = .005) and 30 N (2.6 ± 3.4 mm vs. 5.3 ± 4.2 mm, P =.044) and in 20% glenoid defects at all translation forces (20 N: –3.2 ± 4.7 mm vs. 0.8 ± 4.1 mm, P = .024; 30 N:–0.9 ± 5.3 mm vs. 4.0 ± 5.2 mm, P = .005; 40 N: 2.1 ± 6.6 mm vs. 6.0 ± 5.7 mm, P = .035).[45] However, similar to previous biomechanical observations regarding isolated conjoint tendon transfer in 20% glenoid defects, DAS leads to a relevant posterior and inferior shift of the humeral head in the abduction external rotation (ABER) position and to a relevant increase in inferior glenohumeral translation and should consequently not be used for large bony defects.[45][88] A comparative study on a subcritical bone model reported significantly improved peak resistance force to anterior displacement when augmenting labral repair with a transfer of the long head of the biceps compared to the conjoint tendon (54.1 ± 5.5 N vs. 46.5 ± 7.6 N; P = .039).[89] The DAS does not appear to limit postoperative rotational range of motion.[45]
Scapular morphology
Specific acromial morphology in the sagittal plane is significantly associated with the direction of glenohumeral instability. In shoulders with posterior instability, the acromion is situated higher and is oriented more horizontally than in shoulders with anterior instability. This acromial position may provide less osseous restraint against posterior humeral head translation. Posterior instability virtually never occurs with a steep ‘Swiss chalet rooftype’ acromion.[90]
Restoration of stability
It is important to keep in mind that even if shoulder stabilization procedures are efficient to prevent recurrent macro-instability (defined as a recurrent shoulder dislocation), they seem inefficient in preventing micro-instability (defined as residual humeral head translation), which could be an explanation for persistent apprehension.[91]
Rehabilitation
From a biomechanical point of view, rehabilitation protocols after glenohumeral instability should avoid excessive pressure and over tensioning on the repaired structures. Regarding pressure, humeral cartilage and labral compression evaluated by motion simulation only occurred in the superior half of the glenoid during exercises.[92] This indicates that postoperative exercises do not lead to important pressure changes on an inferior labral repair. Concerning soft tissue tension, rehabilitation should be performed in the scapular plane, which lies about 30 degrees anterior to the coronal plane of the body.[93] This position allows for decreased stress on the anterior capsular structures, optimized glenohumeral congruence and enhanced functional activity of the posterior cuff compared to the body plane.[93]
The rotator cuff acts as a key dynamic stabilizer, and if its force couples go unbalanced, the deltoid muscle will create an upward migration of the humeral head and secondary cuff impingement.[94] The same principle applies to the scapula, where the serratus anterior and trapezius act as the primary force couple stabilizing the scapula in abduction in the scapular plane.[95] Rehabilitation should therefore focus on strengthening and careful balancing of these force couples. Regarding soft tissues repair, protection is best achieved by avoiding constraints to the antero-inferior capsule-labral complex. At 0 degree of abduction, Black et al. found that the low-tension zone was around 45 degrees of external rotation, in case of anterior capsular shortening of only 2 mm this zone was reduced by an additional 20 degrees.[96] Penna et al. confirmed these findings, further reporting that combination of passive abduction and external rotation was responsible for a maximum measured force of 17.7 N on a capsule-labral repair.[97] While it seems reasonable to limit excessive stress on the capsule during early rehabilitation, residual capsular shortening on the other hand should be avoided as it alters physiologic glenohumeral head translation.[98]
Rotator cuff
The physiologic state
The role of the rotator cuff is to work in conjunction with the deltoid to balance the force couples around the glenohumeral joint. In the horizontal plane, the cross-sectional area and force couples between the anterior (subscapularis) and posterior (infraspinatus and teres minor) rotator cuff are balanced.[99] The forces generated by the subscapularis, the supraspinatus, the infraspinatus and the teres minor are 53%, 14%, 22% and 10% respectively.[99] The subscapularis seems to be a key muscle for anterior forward flexion,[100] while the infraspinatus prevents superior and anterior translation of the humeral head.[101]
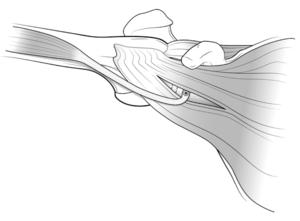
The rotator cable, first described by Burkhart et al.[102] as a thick bundle of fibres perpendicular to the supraspinatus, is of major biomechanical importance (Figure).[103] It is mandatory to have a good understanding of the anatomy surrounding the rotator cable as well as the close relationship between the insertion of the supraspinatus and infraspinatus tendons as well as the coracohumeral ligament. The rotator cable outlines the rotator crescent which is a relative avascular lateral portion of the supra and infraspinatus tendons. The anterior cable inserts in close relation to the coracohumeral ligament into the anterior greater tuberosity and upper lesser tuberosity, representing fibres of the anterior supraspinatus. The posterior cable insertion will be located at the junction between the infraspinatus and teres minor.[103][104] Thus, a tear involving all of the infraspinatus disrupts the posterior cable while disruption of the anterior cable requires a tear involving the upper half of the subscapularis tendon. The function of the cable is frequently compared to that of a suspension bridge which transmits the forces of the cuff through the span to its pillars. This mechanism could explain why function is preserved in tears involving only the rotator crescent (Figure) and why partial cuff repairs with restoration of the pillars can restore good function.[103][105]
Further, anatomical pseudoparalysis (defined as the inability to actively forward elevate the arm > 90 degrees with complete passive anterior forward elevation) was shown to be the consequence of the disruption of at least one rotator cable attachment, subsequently leading to insufficient equilibrium in the vertical plane and resulting in altered kinematics.[106] Bouaicha et al. recently introduced the concept of the shoulder abduction index (SAM), which is basically a ratio of the lever arm of the rotator cuff and deltoid as an anatomic predictor to the appearance of pseudoparalysis.[107] According to their work, a SAM < 0.77 (odds ratio 11) in the presence of a massive rotator cuff tear is predictive of pseudoparalysis.
Rotator cuff tear repair
It appears preferable to restore the anatomy of the rotator cuff after a tear whenever possible to restore load transmission from tendon to bone. This can, however, be challenging when facing large and retracted tear patterns, particularly chronic tears. A medially non-anatomic reinsertion significantly reduces the compressive glenohumeral joint reaction forces, the glenohumeral stability and the supraspinatus moment arm, especially in abduction.[108] Consequently, medialization of the supraspinatus should be limited to 10 mm as it does not seem to limit shoulder range of motion by internal impingement.[109][110] Denard et al. reported that subscapularis footprint medialization by up to 4 to 7 mm is also functionally acceptable.[111] Articular-sided rotator cuff tears are thought to be the equivalent of superior capsular rupture and a physiological adaptation in the throwing athlete allowing enhanced external rotation and anterior humeral translation.[112][113] However, biomechanical studies have shown that a partial-thickness tear will lead to altered strain patterns in the remaining cuff and therefore enhance the risk of tear propagation.[114][115] A trans-tendon repair of articular-sided partial-thickness rotator cuff tears was shown to reduce glenohumeral contact pressure and contact area during internal impingement but also subacromial contact pressure.[116] The latter assumes that the repair is done without overtensioning.
The coracoacromial arch
Another important point is that contact between the rotator cuff and the coracoacromial arch is not per definition a pathologic state and can be seen under physiologic conditions.[117] While acromion shape has been the source of extensive research, an increased critical shoulder angle (38 degrees) has been pointed out as a source of increased load to the supraspinatus tendon at lower degrees of abduction.[118] This led to the suggestion to perform a lateral acromioplasty instead of anterior subacromial decompression as an adjunct to rotator cuff repair.[119] This further has the advantage of preserving the acromial insertion of the coracoacromial ligament which, when resected, allows anterosuperior humeral head translation.[120]
Surgical possibilities in case of irreparable rotator cuff lesions When facing impaired shoulder function in the presence of an irreparable postero-superior cuff tear, several surgical options have been proposed. Tendon transfers, commonly using the latissimus dorsi and more recently the lower trapezius can both significantly enhance shoulder function. While the main goal of the tendon transfer is to restore external rotation, recent biomechanical data favours the use of lower trapezius tendon transfer to the infraspinatus insertion because of both stronger abduction and external rotation moment arms.[121] The development of arthroscopic surgery led to an increased awareness and subsequently better understanding of the superior capsule, which is closely related to the undersurface of the supraspinatus and infraspinatus tendons and resists superior migration of the humeral head.[122] Subsequent research showed that a double-layer repair with inherent approximation of the superior capsule leads to improved biomechanical properties of the construct.[123] In the setting of an irreparable cuff, superior capsular reconstruction (SCR) using either an autograft (tensor fascia lata),[124] a dermal allograft[125] or the long head of the biceps[126] recreates a passive restraint to superior and anteroinferior translation.[127] Therefore, adding a static stabilization like the SCR to a dynamic stabilizer like a tendon transfer may ultimately enhance articular stability at the low to mid ranges of abduction.[128] Finally, SCR is a promising procedure that remains, however, relatively new and is subject to further research regarding optimal graft choice and surgical technique to avoid excessive strain on the construct during activities of daily living.[129][130]
The last proposed solution trying to restore glenohumeral contact pressures is the implantation of a balloon spacer in the subacromial space. In a recent cadaveric study, this procedure was shown to efficiently lower the humeral head, increase deltoid load and normalize articular contact pressure at most abduction angles.[131] While the use of a biodegradable balloon may be questionable regarding long-term outcomes in the setting of an irreparable tear, it could on the other hand be a suitable adjunct to rotator cuff repair by reducing peak pressure and wear on the repair, potentially avoiding a re-tear.[132]
An irreparable isolated subscapularis tear implies not solely a tendon failure, but also rupture of the underlying anterior capsule and ligaments, leading to subsequent altered shoulder kinematics. The biomechanical specificity being that both a dynamic and static stabilizing force is impaired, consequently increasing anterior and inferior humeral head translation.[133] Treatment options include tendon transfer of the pectoralis major or latissimus dorsi tendon and/or anterior capsule reconstruction.[134] An in vitro study by Konrad et al reported increased restoration of humeral head translation when the pectoralis tendon was transferred behind the conjoint tendon, allowing better restoration of the line of action of the subscapularis tendon.[135] This led to further anatomic studies favouring an anterior transfer of the latissimus dorsi tendon.[136] A variety of options have been proposed for anterior capsule reconstruction including autografts (tensor fascia lata, hamstrings), tendon allograft, or human dermal allograft.[137] A cadaveric study by Komperda et al. revealed that anterior capsular reconstruction was superior to pectoralis major tendon transfer to restore anterior and inferior humeral head translation.[137] Further, the addition of an anterior latissimus dorsi tendon transfer to an anterior capsular reconstruction did not enhance antero-inferior humeral head stability.[133]
Stiffness
Joint stiffness is characterized by the limitation of both active and passive motion with only strength remaining normal. On top of the retracted capsule, adhesions in the subacromial and subdeltoid space (i.e after trauma or surgery) will lead to a loss of tissue compliance and impede motion. Frozen shoulder is a condition characterized by thickening of the joint capsule and presence of adhesions in the anterior capsule and axillary pouch that creates a significant reduction of joint volume.[138][139] As mentioned earlier, the glenohumeral ligaments represent capsular thickening and are physiologically only tight at the end points of range of motion. The thin capsule is adherent to the rotator cuff except in the rotator interval and axillary pouch. While the inferior glenohumeral is thought to limit external rotation in abduction, the rotator interval (including superior glenohumeral and coraco-humeral ligament) limits external rotation with the arm in adduction.[140][141] Finally, isolated limitation of internal rotation is thought to be due to the posterior capsule.[140] However, when performing arthrolysis, section of the inferior glenohumeral ligament alone do not restore internal rotation. An additional section of the coracohumeral ligament is necessary. Scapulothoracic motion who represent one third of total shoulder elevation becomes crucial to compensate for glenohumeral stiffness and is known to be also altered in this setting.[142]
Rehabilitation
The primary goal of cuff repair is to be as anatomic as possible and to create a biomechanically favourable environment for tendon healing. Rehabilitation protocols must logically be adapted to the strength of the repair and tissue quality. Basic science research has mainly focused on the effect of mechanical loading on tendon-to-bone repair during the acute phase of healing using rat models.[143] While some authors reported improved tendon-to-bone healing with immobilization,[143][144] others have found that limited early (during the first six weeks after a repair) tensile load is beneficial for viscoelastic tendon properties.[145][146] However, uncontrolled tensile load (as seen with open chain exercises, eccentric muscle activation and motion beyond repair elasticity), leads to impaired tissue healing and can predispose to re-tear or repair tissue elongation.[147][148][149] Excessive compressive loads, typically increased by postoperative scapular protraction,[150] do further impair tissue healing.[143][151] Lastly, Sonnabend et al., in a primate model, reported that while eight weeks after cuff repair the tissue appeared macroscopically healed, mature healing with Sharpey fibres started at 12 weeks, therefore supporting a 12–15 week rehabilitation programme.[152] Further studies are needed to provide guidelines for rehabilitation based on tear size and type of repair.
Reproduced from Goetti et al., with permission.[2]
Anatomic total shoulder arthroplasty
Anatomy is key to successfully reproduce patient’s physiologic joint kinematics. By virtue of its mobility, the glenohumeral joint is predisposed to instability. One factor affecting stability is the radius of curvature mismatch between the humeral head and glenoid. Further, only 20 to 30% of the humeral head is in contact with the glenoid.[153] The rotator cuff acts as an essential dynamic stabilizing force centering the humeral in the mid-portion of range of motion and is crucial for an anatomic total shoulder arthroplasty to be effective.[12] The supraspinatus helps to center the humeral head against the force of the deltoid in lower degrees of abduction, while the infraspinatus and teres minor help to clear the greater tuberosity under the coraco-acromial arch when the arm is moved in abduction and external rotation.[12][154] Lastly, even though the shoulder is not a weight-bearing joint, joint reaction forces as high as 2,4 times body weight have been reported during shoulder rehabilitation.[155]
Humeral head
Proximal humerus anatomy is subject to great variability, which is further significantly modified by arthritic changes.[156][157] As anatomic total shoulder arthroplasty can restore physiologic shoulder kinetics, a thorough knowledge of normal anatomy appears mandatory as one cannot simply rely on perioperative measures (Figure).[158] The non-arthritic humeral head has a mean three-dimensional measured diameter of 46.2 + 5.4 mm (range, 37.1 to 56.9 mm) and a humeral height of approximately 19 mm (Figure).[159][160][161][162][163]
The osteoarthritic head is flattened and widened with a mean diameter of 59 ± 9 mm.[156] The humeral head has the particularity to be elliptic in the periphery and become spherical in its central part, meaning that the cut surface will be about 2 mm larger from medial to lateral than from anterior to posterior.[163] While spherical humeral head implants are mainly used in shoulder arthroplasty, elliptic implants have been proposed to reproduce anatomy and theoretically improve the rotational range of motion. The ratio between humeral head size and height is relatively constant.[164] The highest point of the humeral head lies 8 + 3.2 mm above the greater tuberosity (Figure).[163]
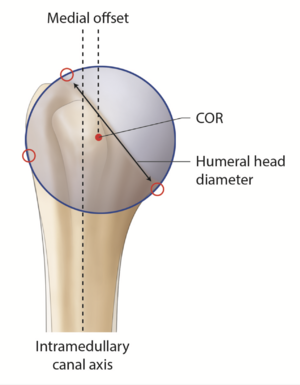
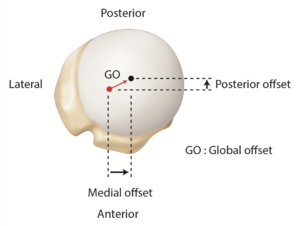
Lastly, relative to the humeral canal, the head has a posterior and medial offset of 0.35 to 2.6 mm and 5.6 to 9.7 mm, respectively (Figures).[165][166]
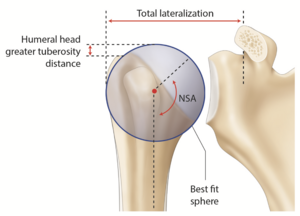
These parameters are helpful to select the appropriate humeral head implant, as this crucial step will ultimately determine the joint center of rotation. However, current biomechanical data does not support significant superiority of the elliptic design over the spherical one regarding the range of motion in internal and external rotation.[167] Terrier et al. illustrated in a numerical shoulder model that a 5 mm malposition of the humeral head implant resulted in impingement or subluxation for an inferior or superior shift, respectively. Both resulted in increased stress on the cement mantle.[168] While joint center of rotation can be determined three-dimensionally by a best-fit sphere using preserved non-articular landmarks, this technique has been translated to a two-dimensional process to allow intraoperative as well as postoperative radiographic evaluation (Figures).[157][169]
However, there is no consensus on cut-off values for joint center of rotation modification, as values as low as 2.5 mm can have been reported to impact impingement free range of motion.[170] Further, if the humeral head is implanted 5 mm too high in regard to the tuberosity, shoulder function will not solely be impaired by a 4 mm decrease in infraspinatus and subscapularis lever arms but also by the tight inferior capsule.[171] Cadaveric studies revealed that an increased humeral component sizing (commonly called “overstuffing”) would modify the center of rotation and add stress to the rotator cuff (Figure). Overstuffing not only decreases shoulder range of motion but also changes rotator cuff lever arm exposing patients to the potential risk of secondary cuff failure.[172][173] Restoring physiologic soft-tissue tension will provide stability and prevent complications such as aseptic loosening and osteolysis induced by stress shielding.[174] Lastly, controversy exists regarding the superiority of resurfacing humeral head over stemmed implants to reproduce physiological shoulder biomechanics.[157][175]
Neck shaft angle
The mean neck-shaft angle or inclination of the proximal humerus is approximately 135 degrees but varies between 115 and 148 degrees (Figure). A study of 2058 humeri by Jeong et al. note that 22% are either < 130 degrees or > 140 degrees.[176] Thus, fixed neck shaft angle humeral stems rely on surgeons to adapt their surgical techniques to accommodate patient anatomy. Modern modular systems provide centered and eccentric humeral heads as well as multiple neck-shaft angle options.
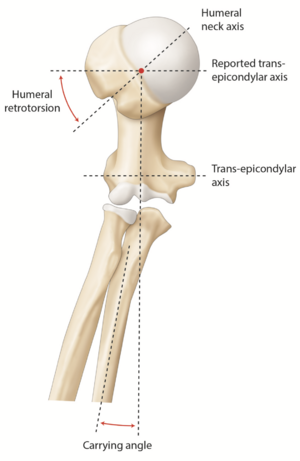
Humeral torsion
Humeral head torsion is important in anatomic total shoulder arthroplasty as it directly affects joint center of rotation and thereby influences mobility in external rotation and shoulder stability.[177][178][179] A cadaveric study by Pearl and Volk reported a mean humeral retrotorsion of 29.8 degrees with a 95% confidence interval of 7 to 52 degrees (Figure).[180] While they used the trochlear axis as a reference, other reported values were based on the transepicondylar axis (which differs from 3 to 8 degrees). Furthermore, current systems use a jig aligned on the forearm as a reference, in this case, a 10 to 15 degrees (carrying angle) must be added to the reported values (Figure). When using a stem with lateral fins, another reliable landmark is to place it 12 + 4 mm behind the bicipital groove.[181] It should, however, be emphasized that the groove rotates about 16 + 7 degrees and appears therefore as an unsuitable landmark in fracture or posttraumatic cases.[182] Lastly, Raniga et al. reported that in Walch B type glenoids, humeral retrotorsion is significantly lower compared to none-arthritic shoulders (14 + 9 degrees vs. 36 ± 12 degrees, p<0.001), suggesting a potential correlation between humeral retrotorsion and glenoid retroversion.[183]
Glenohumeral offset
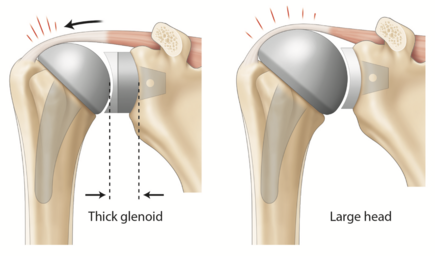
Osteoarthritis results in loss of glenohumeral offset secondary to humeral and glenoid bone wear. While glenohumeral offset is subject to inter-person-variability, a diminished glenohumeral offset implies altered deltoid and rotator cuff moment arms, as well as modified capsular tension (Figure).[159][163] This is thought to influence the postoperative range of motion by limiting active abduction as well as creating a tendency to inferiorly sublux the humeral head.[178][184] Conversely, thick glenoid components create overstuffing (Figure). Bodrogi et al. recently described a reliable CT-based method to assess changes between pre and post-arthroplasty glenohumeral offset measures.[185] In the absence of humeral head sphericity (particularly in the setting of osteoarthritis), their method relied on the center of the humeral shaft (rather than the center of the humeral head) as described by Jacobsen and Friedman’s line to be independent of retroversion on the glenoid side.[186]
Medullary canal
Finally, the intramedullary canal not only becomes tighter but also increasingly retroverted from proximal to distal.[162] Fixation of the humeral component is widely varied. Diaphyseal press-fit stems induce proximal stress shielding. Cementation is reliable at time zero but difficult in revision. The goals of reduced stress shielding, easier stem revision, and preservation of vascularity have led to a progressive shift towards short metaphyseal stem or stemless fixation.[174] While a comparative cadaveric study revealed decreased micromotion and enhanced rotational stability in cemented stems,[187] optimal stem fixation, length, and filling ratio to avoid stress shielding,[188] subsidence,[189] and misalignment remains controversial.[190]
Glenoid anatomy
Glenoid loosening remains the primary cause of anatomic total shoulder arthroplasty failure.[191] Similar to the humeral side, osteoarthritis appears to modify normal glenoid anatomy significantly. The glenoid seems relatively small and shallow compared to the humerus, with only 9 cm2 of articular surface.[192] The glenoid is pear-shaped with a superior to an inferior dimension of 39 mm an inferior glenoid width averaging 29 mm.[163] There is a radii mismatch between the glenoid and humeral head, while the radius of curvature is greater in the anteroposterior than the superoinferior direction (41 vs. 32 mm).[153] Biomechanically, perfect conformity leads to a more stable joint but increased stress on the glenoid. On the other hand, an increased mismatch in radii will lead to increased translation of the humerus onto the glenoid with rim loading of the glenoid component causing a “rocking horse” effect.[193][194] Based on current techniques, the best compromise appears to be a mismatch ranging between 4 and 8 mm.[195] However, it should be noted that these findings are based on a spherical humeral head. It has been proposed that conformed designs are better suited for elliptical heads.[196]
Glenoid version and inclination
Reported three-dimensional CT-derived measures report mean normal glenoid retroversion of 6 ± 4 degrees and inclination of 7 ± 5 degrees. Retroversion has been correlated (r = 0.7, P < 0.001) to posterior humeral head subluxation (59% ± 7%).[197] The contralateral shoulder may be a reliable model, like side to side differences are limited to 5 degrees in 95% of the cases.[198] It is also important to assess the version in three dimensions, as in cases with >10 degrees version, it is not solely direct posteriorly but also in superior, inferior, and anterior directions.[199] A further important hint when performing anatomic total shoulder arthroplasty is that the version of the inferior part of the glenoid shows substantial less variability compared to the upper part and should therefore be used as the preferred intra-operative landmark in order to achieve adequate implant positioning.[200]
Concerning inclination, Moor et al. proposed the critical shoulder angle as a measure of scapular morphology with the benefit of combining measurements of glenoid inclination and lateral acromion coverage.[201] They identified an angle inferior to 30 degrees as being associated with primary shoulder osteoarthritis. This finding is supported by subsequent biomechanical studies reporting increased joint reaction forces in case of a lower critical shoulder angle.[202][203] Critical shoulder angle >35 degrees is, on the other hand, related to an increased incidence of rotator cuff tears secondary to increased supraspinatus loading to compensate for increased joint instability as a consequence of increased glenohumeral joint shear forces.[201][204][205] In the setting of anatomic total shoulder arthroplasty, an increased critical shoulder angle has been related to an increased incidence of glenoid radiolucencies.[205]
Humeral head subluxation
The Walch classification, with subsequent modifications, is the most common means of assessing glenoid changes secondary to primary osteoarthritis.[206][207] Walch classified glenoid deformity based on posterior glenoid retroversion and humeral head subluxation. In opposition to type A glenoids (symmetrical bone loss), type B glenoids (asymmetrical bone loss) have been associated with progressive posterior glenoid bone loss over time.[208] This factor is important when evaluating posterior humeral head subluxation; in type B3 glenoids, the head might be centered in regard to the glenoid but be posteriorly translated in relation to the scapula. Iannoti et al., by using three-dimensional standardized measures, reported a continuum of measures among the different type B and C glenoids rather than defined categories (B1, B2, B3, and C) in regard to glenoid retroversion and humeral head subluxation.[209] Currently, it is still debated if posterior humeral subluxation is the cause or consequence of increased retroversion.[210] Static posterior humeral head subluxation and posterior glenoid wear have both been associated with premature osteoarthritis in young men and related to higher complication rates after anatomic total shoulder arthroplasty.[211][212][213][214] Recently, Beeler et al. identified a flat acromion roof as a potential risk factor for posterior humeral head subluxation and posterior glenoid wear.[215] This hypothesis was confirmed by a subsequent study by Meyer et al., reporting a median of 4 degrees more glenoid retroversion and a 5 degrees less steep acromion in type B2 and C compared to type A and B1 glenoids (P ≤ 0.022).[216]
Instability
The rotator cuff and the horizontal force couple are critical to glenohumeral stability.[217] By respecting cuff insertion and restoring bony anatomy, force couples should be adequately restored. Soft tissue balancing, by the combination of the anterior subscapularis tendon and capsule release sometimes associated with a capsulorraphy of the redundant posterior capsule, is indicated to reach Matsen’s criteria (40 degrees of external rotation, 60 degrees of internal rotation and a 50% posterior shift of the humeral head over the glenoid).[218] If bony correction is necessary, one should carefully reevaluate adequate humeral implant size as center of rotation likely changed secondary to the additional bone removal. When facing a retroverted glenoid, posterior instability can be compensated for by anteriorly offsetting the humeral head component, leading to a significant anterior humeral displacement on muscle activation as well as an anterior shift of the center of pressure (p<0.05).[219][220] A major downside of this technique, however, is increased tension on the subscapularis with potentially higher rates of subscapularis failures. Chronic irreparable subscapularis deficiency is a contraindication to anatomic total shoulder arthroplasty as it tends to destabilize the joint secondary to an upward migration of the humeral head and eccentric contact pressure onto the glenoid.[221] While subscapularis preserving approaches have been described, most surgeons access the glenohumeral joint by subscapularis detachment with either a tenotomy, peel, or lesser tuberosity osteotomy. Effective subscapularis repair[222] during surgery is therefore mandatory; a review of biomechanical cadaveric studies suggests superior load to failure for the osteotomy at time zero but no difference at cyclic loading[223][224] While de Wilde suggested that a C-block lesser tuberosity osteotomy might prevent postoperative subscapularis fatty infiltration, a recent systematic review reported no statistical difference in clinical and radiological outcomes between tenotomy, peel and osteotomy.[225][226][227] In case of postoperative rupture, a prompt secondary repair can be considered to prevent instability but has been associated with variable results.[228][229] The addition of anterior latissimus dorsi transfer seems biomechanical superior to the pectoralis major transfer in anatomic total shoulder arthroplasty due to an improved internal rotation moment arm and more similar line of pull relative to the subscapularis.[230]
Glenoid bone loss
Correcting glenohumeral bone loss is an important step when implanting the glenoid component. Implanting the component in excessive retroversion will result in posterior translation of the humeral head and subsequent rim-loading known to cause early component loosening.[231][232] According to a finite element model by Farron et al., 10 degrees of retroversion should be considered as the cut-off value.[233] In their analysis, an implant with 20 degrees of retroversion resulted in a 326% increased stress within the cement mantel and a 706% increase of micromotion at the bone-cement interface. Recent work using statistical shape modeling allowed a computer reconstruction of the premorbid glenoid with a precision of about 1 mm and 2 degrees for version and inclination.[234][235] Several techniques to correct retroversion were developed. If version is corrected alone by means of anterior glenoid reaming, it will lead to significant joint line medialization and central cortex perforation when correction exceeds 15 degrees.[236] Consequently, posterior augmented glenoid implants were developed to avoid the medialization of the joint line, with encouraging early results.[237] However, severe deformity has been associated with loosening of such components.[238]
Proper implantation technique avoiding superior inclination or retroversion is thought to be crucial to avoid edge-loading causing micromotion and subsequent breakdown at the bone-implant interface, ultimately leading to aseptic loosening.[233][239] For the same reason, an intact cuff is also mandatory to conserve physiologic joint kinematics and therefore limit polyethylene wear.[240] While most current anatomic total shoulder arthroplasty heads are metallic, experimental studies suggest that a change towards ceramic heads could reduce polyethylene wear rate by up to 26.7%.[241] A wide range of onlay all-polyethylene glenoid shapes (pear-shaped versus elliptic) and sizes are currently available on the market, with no current consensus on optimal designs regarding back-surface (flat versus curved), anchorage (keel versus peg) or level of conformity.[242] Further, a recent cadaveric study comparing inlay (implanted into the bone socket and therefore allowing for circumferential bone support) with onlay components revealed superior outcome regarding joint reaction forces and fatigue failure in favor of the inlay design.[243] There is also renewed interest towards metal-back glenoids in response to the reported encouraging survival rates of modern designs.[244] While the theoretical benefit of more stable fixation and easy conversion to reverse shoulder arthroplasty seems appealing, long-term outcomes are awaited based on the long list of retrieved pre-existing metal-back designs.[245]
Reverse shoulder arthroplasty
Historically reverse shoulder arthroplasty was developed to address arthritis in cuff deficient shoulders as the loss of dynamic compression provided by the rotator cuff led to instability and early glenoid loosening, therefore resulting in unpredictable outcomes with large head hemiarthroplasty or anatomic total shoulder arthroplasty.[246][247] The reverse ball and socket “Grammont type” reverse shoulder arthroplasty was introduced in 1985 and is based on the biomechanical principles of a medialized joint center of rotation, distalized humerus, and a semi-constrained design with a constant joint center of rotation.[248] On contrary to anatomic total shoulder arthroplasty, in which the humeral head rotates in a spinning motion around itself as the center of rotation lies inside the humeral head, the constant center of rotation in reverse shoulder arthroplasty lies inside the glenosphere and leads to a hinged motion of the humerus, making it prone to impingement thereby limiting range of motion.[249]
Modifications in muscle recruitment
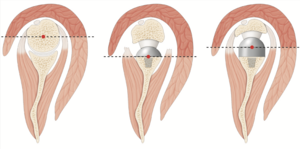
The aforementioned modifications to physiologic shoulder anatomy lead to a 42% increased deltoid lever arm, as well as an increased recruitment of anterior deltoid muscle fibers to perform abduction.[250] The original design with a 155 degrees non-anatomic stem further enhanced the deltoid lever arm by distalization of the humerus.[251] The anterior deltoid becomes consecutively an important contributor to flexion and abduction moment arms.[252] In case of a deficient anterior deltoid (i.e., revision surgery with detached or paretic anterior deltoid)[253] compensation for abduction relies on significantly enhanced force of the subscapularis (195%) and middle portion of the deltoid (26%).[254] There are, however, drawbacks to these anatomic modifications of physiologic moment arms. While the anterior and posterior deltoid as well as pectoralis major are recruited as additional flexors and abductors, the latissimus dorsi, teres major, and lower part of the pectoralis major have increased adductor and extensor moment arms, therefore directly limiting their participation in active internal and external rotation.[255][256] As lever arms of the anterior and posterior cuff are already decreased secondary to humeral medialization, this adds to a further weakening of active internal and external rotation.[257][258] This issue can either be addressed by the addition of a tendon transfer or by modifying the classic reverse shoulder arthroplasty design to a “lateralized” one.[259] This modification will preserve rotational moment arms of the subscapularis and teres minor and therefore enhance active range of motion in the axial plane (Figure).[260] Finally, while the postoperative range of motion takes place inside the prosthetic joint, scapulothoracic participation is significantly increased after reverse shoulder arthroplasty.[261]
Medialization of the joint center of rotation
The biomechanical benefit of a medialized joint center of rotation is to convert torque forces into compressive forces across the bone-glenosphere interface and therefore provide stability and enhanced component integration.[262] As the rotator cuff no longer provides its compressive forces, the fixed center of rotation allows the deltoid to compensate and provide the needed compression to stabilize the joint.[250] While in anatomic total shoulder arthroplasty joint reaction forces can reach up to 90% of body weight at 90 degrees of abduction, reverse shoulder arthroplasty design reduces both compressive and shear stress and therefore joint reaction forces by up to 42%. This further allows active abduction with a 20% decreased deltoid activity in a cuff deficient shoulder.[263][264][265]
There is, however, a major drawback of center of rotation medialization, in the form of impingement between the scapular neck and humeral prosthetic component defined as scapular notching.[266][267] Several technical factors improve impingement free range of motion. One option is placing the glenosphere (not the baseplate) below the inferior glenoid rim or using an inferior eccentric glenosphere.[268] De Wilde et al. et reported that a 5 mm overhang could improve impingement free adduction by 39 degrees.[269] Abduction is also positively correlated with acromiohumeral distance (r = 0.93; p < 0.001) which is increased with an eccentric glenosphere.[270] The ideal amount of overhang relative to the glenoid appears to be about 2.5 mm based on clinical evidence.[271] Alternatively, glenosphere diameter can be increased, therefore upsizing the diameter from 38 to 46 mm was reported to not only increase range of motion by 39% but also stability by a 36% increase in jump distance.[272] Accenter of rotationding to a computer simulation of impingement free range of motion, the single most effective modification in prosthetic design is the change of humeral neck-shaft angle from the classic 155 towards a more anatomic angle.[273][274]
While joint center of rotation needs to be medialized in regard to the native center of rotation, slight lateralization of the glenosphere from the glenoid can further enhance compressive forces, which are thought to overcome the increased shear forces at the bone-component interface.[262] Basic science studies show several benefits of lateralization. In both sawbone[275] and computer models,[273][276][277] lateralization improves range of motion in all directions.[277] There is an ongoing debate regarding the impact of lateralization on the risk for acromial stress fractures. Finite element analysis has suggested a 17,2% increased acromial stress secondary to 10 mm lateralization.[278] Clinically, distalization has been implicated as more of a culprit than lateralization.[279] Glenosphere lateralization has further a linear correlation with baseplate micromotion[280] and therefore exposes to the risk of aseptic loosening.[281] Giles et al. tested the effect of glenoid and humeral lateralization on deltoid muscle load in vitro using a simulator. They reported that 10 mm of humeral lateralization was the only parameter that actually decreased deltoid force in abduction (65 ± 8%), however, warned that this benefit may not compensate for the negative effects induced by glenosphere lateralization.[282] Lastly, Boileau et al. proposed a bony increased-offset reverse shoulder arthroplasty to lateralize the glenosphere however maintaining center of rotation at the prosthesis-bone interface and thereby minimizing torque stress.[283]
Baseplate design
To allow bone ingrowth, baseplate micromotion must be inferior to 150 um.[284] As baseplates are screwed down to the glenoid, research focused on the optimal configuration to enhance initial stability on polyurethane foam models. While increased screw length (>17 mm inside the glenoid) or screw diameter (3.5 vs. 5.0 mm) was shown to additionally reduce micromotion by up to 30%, inclining screws by 30 degrees (compared to 0 degree) was the most effective as it led to a 50% reduction of micromotion.[262][285] With a central post design, the most important screw in the baseplate is thought to be the inferior one, as tensile forces are the highest at the inferior border secondary to humeral loading. A locking screw should therefore be favored in this particular location as a 7% enhanced load to failure was reported compared to standard cortical screws.[286] Regarding the total number of screws, a cadaveric study comparing a two peripheral screws flat-backed baseplate construct (superior and inferior one) with a four screws construct found no statistical difference regarding motion during cyclic loading.[287] Regarding baseplate design, the central screw does not seem superior to the post regarding load to failure compared to the central post.[288] Lastly, Gutierrez et al. investigated optimal baseplate position using a computer model. According to their work that focused on uniform force distribution, a 15 degrees inferior tilt is best suited for concentric or lateral eccentric glenosphere, for inferior eccentric glenosphere a neutral inclination (0 degree) is the preferred orientation.[289][290] Superior tilt should always be avoided as stress at the bone interface increases. Boileau et al. suggested that superior tilt is commonly underestimated during reverse shoulder arthroplasty planification.[291] As the baseplate is implanted in the inferior part of the glenoid, they introduced the reverse shoulder arthroplasty angle, defined as the angle between the inferior part of the glenoid fossa and the perpendicular to the floor of the supraspinatus. Compared to the anatomic total shoulder arthroplasty angle (β angle or global glenoid inclination angle), the reverse shoulder arthroplasty angle is 8 ± 4 degrees larger.
Stability
The stabilizing effect of the rotator cuff is inexistent in a cuff-deficient shoulder, making it prone to instability.[251] In the physiologic state, the glenoid serves as a pillar for the humeral head. During shoulder range of motion, combined physiologic glenohumeral and scapulohumeral motion keep this pillar beneath the humeral head. Altered muscle balance forces in cuff tear arthropathy shoulders disrupt this dynamic process and explain the eccentric wear pattern encountered in cuff tear arthropathy. The endpoint is reached when the humeral head migrates upward and creates an acetabularization of the acromion, allowing a neutralization of the dynamic instability.[292]
Instability is one of the most cited complications after reverse shoulder arthroplasty.[293] A wide variety of actors potentially influence stability, these including glenosphere (eccentricity, diameter, inclination), humeral socket depth, humeral implant version, as well as humeral lateralization and length, as well as remaining subscapularis.[289][293][294][295][296] The arm position most prone to instability is 30 degrees of abduction with neutral or internal rotation.[296] Increasing glenosphere diameter from 38 to 42 mm was reported to augment stability by 32% by increasing joint load and deltoid force.[296][297] Glenopshere positioning will impact stability as a 2 mm inferior offset enhance stability by 17%.[269][293] Biomechanical data also suggests that superior tilt exposes to a higher risk of instability.[268][298] Glenosphere lateralization is effective to prevent scapular impingement with the arm in adduction and to increase the force needed for anterior dislocation, the biomechanical benefit of a reduced deltoid force to abduct the arm is unfortunately lost (with lateralization of 15 mm).[299][300] Comparison of humeral neck-shaft angle (135 vs.155 degrees) revealed only a minor benefit with higher dislocation forces required in 135 degrees stems at 30 degrees of abduction; this effect was however negligible compared to a 6-9 mm glenoid lateralization.[299] Avoiding excessive humeral retrotorsion (>10 degrees) seems to have a higher impact on stability than glenosphere retroversion (>20 degrees).[301] Conformity in radii between the glenosphere and humeral socket present in reverse shoulder arthroplasty results in an enhanced joint-reaction force vector tolerance to up to 45 degrees (compared to 30 degrees in the setting of an anatomic total shoulder arthroplasty).[280] Lastly, humeral socket depth defined in ratio to glenosphere diameter will increase stability at the potential cost of a reduced range of motion.[289][294][302]
Distalization of the humerus
While distalization of the humerus is a central point in reverse shoulder arthroplasty with the primary goal of increasing the lever arm of the deltoid and improving functional outcomes, there are consequences to lengthening. Optimal lengthening is thought to be around 2 cm but is still debated.[303] While insufficient lengthening (particularly in the revision setting) has been shown to be a critical factor regarding joint instability,[303][304] downsides of excessive lengthening include increasing the risk of a neurological lesion (neurapraxia) and over-tensioning resulting in a decreased range of motion as well as increased joint reaction forces.[305][306] Furthermore, lengthening via an onlay humeral component has been associated with an increased risk of acromial stress fracture compared to inlay components.[279] While there is no current consensus regarding the optimal way to increase soft-tissue tension while avoiding complications,[294][307] recent biomechanical data suggests that humeral lateralization could potentially be a solution to improve joint and muscle loading.[282][308][309] However, one must keep in mind that humeral lateralization also leads to distalization. In addition to the aforementioned consequences, distalization also changes the force vectors of the remaining rotator cuff. The latter may be particularly important in the use of reverse shoulder arthroplasty for diagnoses other than rotator cuff arthropathy in which much of the rotator cuff is still functional such as primary glenohumeral arthritis with posterior subluxation and a biconcave glenoid. Thus, there are not only trade-offs to distalization, but the ideal amount may also vary by diagnosis.
Conclusions
The shoulder is a complex biomechanical entity with close relationships between anatomical structures and the biomechanical consequences of the different pathologies encountered. Soft tissue stabilizers, bone morphology, and dynamic stabilizers such as the rotator cuff and long head of the biceps tendon all interact to ensure shoulder stability. Balanced glenohumeral and scapular force couples are mandatory to preserve or restore shoulder function. Further, a thorough knowledge of anatomy and biomechanical properties of the rotator cuff, underlying joint capsule, rotator cable, and coracoacromial arch is essential when performing a rotator cuff repair. The huge potential of the human body to cope and adapt to the different pathologies can make it sometimes challenging to differentiate between an anatomical or pathological variant. The wide range of pathologies encountered as well as the even higher number of proposed anatomic and nonanatomic surgical solutions make it a very interesting subject for further research. The understanding of the discussed biomechanical principles should therefore be of great help to the surgeon treating these pathologies.
As the number of primary and revision shoulder arthroplasty is thought to progress by up to 322% by 2050, a thorough understanding of the biomechanical principle seems mandatory. The key concepts between these two procedures are yet very different. While reproducing anatomy is at the center of anatomic total shoulder arthroplasty philosophy. Therefore, a thorough understanding of premorbid anatomy is crucial to success, as inadequate restoration of the joint center of rotation will predispose to secondary cuff failure and glenoid implant loosening. Further, posterior glenoid bone loss and humeral head subluxation (typically seen in Walch B2 and C glenoids) should be corrected to avoid premature glenoid component failure. While posterior augmented anatomic glenoid implants might solve this issue in the near future, a shift towards reverse shoulder arthroplasty in this particular setting can already be observed. With its semi-constraint design, reverse shoulder arthroplasty was initially developed to treat cuff tear arthropathy patients. Current indications further expanded towards primary OA with glenoid dysplasia, irreparable rotator cuff tears, three- and four-part fractures as well as revision of failed anatomic total shoulder arthroplasty. The main complication with the original Grammont design is scapular notching, which might lead to secondary glenoid loosening. Inferior baseplate positioning and therefore inferior glenosphere overhang, bony or metallic baseplate lateralization as well as avoiding superior inclination, all minimize the risk of scapular impingement. Lower humeral neck-shaft angles can further reduce the risk of scapular notching and might enhance deltoid muscle recruitment and cuff tension, thereby potentially improving active external rotation. Current research on optimal reverse shoulder arthroplasty design focuses on improved impingement-free range of motion. However, increased range of motion should not be made at the cost of decreased stability or scapular fractures. One should always keep in mind that the goal of every arthroplasty is to alleviate pain and restore the best possible function.
References
- ↑ Goetti P, Denard PJ, Collin P, Ibrahim M, Hoffmeyer P, Lädermann A. Shoulder biomechanics in normal and selected pathological conditions. EFORT Open Rev. 2020;5(8):508-518
- ↑ 2.0 2.1 Goetti P, Denard PJ, Collin P, Ibrahim M, Mazzolari A, Lädermann A. Biomechanics of anatomic and reverse shoulder arthroplasty. EFORT Open Rev In Press
- ↑ Burkhart SS, Lo IK. Arthroscopic rotator cuff repair. J Am Acad Orthop Surg 2006;14:333–346
- ↑ Vidt ME, Santago AC, II, Marsh AP, Hegedus EJ, Tuohy CJ, Poehling GG, Freehill MT , Miller ME , Saul KR. Modeling a rotator cuff tear: individualized shoulder muscle forces influence glenohumeral joint contact force predictions. Clin Biomech (Bristol, Avon) 2018;60:20–29
- ↑ Williamson P, Mohamadi A, Ramappa AJ, DeAngelis JP, Nazarian A. Shoulder biomechanics of RC repair and instability: a systematic review of cadaveric methodology. J Biomech 2019;82:280–290
- ↑ Saul KR, Hu X, Goehler CM, Vidt ME, Daly M, Velisar A, Murray WM. Benchmarking of dynamic simulation predictions in two software platforms using an upper limb musculoskeletal model. Comput Methods Biomech Biomed Engin 2015;18:1445–1458
- ↑ Nikooyan AA, Veeger HE, Westerhoff P, Graichen F, Bergmann G, van der Helm FC. Validation of the Delft Shoulder and Elbow Model using in-vivo glenohumeral joint contact forces. J Biomech 2010;43:3007–3014
- ↑ 8.0 8.1 8.2 8.3 Stimec BV, Lädermann A, Wohlwend A, Fasel JH. Medial coracoclavicular ligament revisited: an anatomic study and review of the literature. Arch Orthop Trauma Surg 2012;132:1071-5 Cite error: Invalid
<ref>tag; name ":1" defined multiple times with different content - ↑ Moya D, Poitevin LA, Postan D, Azulay GA, Valente S, Giacomelli F, Mamone LA. The medial coracoclavicular ligament: anatomy, biomechanics,and clinical relevance-a research study. JSES Open Access. 2018 Sep 22;2(4):183-189
- ↑ Weaver JK, Dunn HK. Treatment of acromioclavicular injuries, especially complete acromioclavicular separation. J Bone Joint Surg Am 1972;54:1187-94.
- ↑ Costic RS, Labriola JE, Rodosky MW, Debski RE. Biomechanical rationale for development of anatomical reconstructions of coracoclavicular ligaments after complete acromioclavicular joint dislocations. Am J Sports Med 2004;32:1929-36.
- ↑ 12.0 12.1 12.2 Mazzocca AD, Santangelo SA, Johnson ST, Rios CG, Dumonski ML, Arciero RA. A biomechanical evaluation of an anatomical coracoclavicular ligament reconstruction. Am J Sports Med 2006;34:236-46 Cite error: Invalid
<ref>tag; name ":4" defined multiple times with different content - ↑ Fukuda K, Craig EV, An KN, Cofield RH, Chao EY. Biomechanical study of the ligamentous system of the acromioclavicular joint. J Bone Joint Surg Am 1986;68:434-40.
- ↑ Abrassart S, Gagey O, Hoffmeyer P. La chape trapézo-deltoïdienne : réalité ou illusion d’optique. Revue de Chirurgie Orthopédique et Réparatrice de l'Appareil Moteur 2007;93:96-7.
- ↑ Lädermann A, Gueorguiev B, Stimec B, Fasel J, Rothstock S, Hoffmeyer P. Acromioclavicular joint reconstruction: a comparative biomechanical study of three techniques. J Shoulder Elbow Surg 2013;22:171-8.
- ↑ Yoo YS, Tsai AG, Ranawat AS, et al. A biomechanical analysis of the native coracoclavicular ligaments and their influence on a new reconstruction using a coracoid tunnel and free tendon graft. Arthroscopy 2010;26:1153-61.
- ↑ Debski RE, Parsons IMt, Woo SL, Fu FH. Effect of capsular injury on acromioclavicular joint mechanics. J Bone Joint Surg Am 2001;83-A:1344-51.
- ↑ Costic RS, Labriola JE, Rodosky MW, Debski RE. Biomechanical rationale for development of anatomical reconstructions of coracoclavicular ligaments after complete acromioclavicular joint dislocations. Am J Sports Med 2004;32:1929-36.
- ↑ Mazzocca AD, Spang JT, Rodriguez RR, et al. Biomechanical and radiographic analysis of partial coracoclavicular ligament injuries. Am J Sports Med 2008;36:1397-402.
- ↑ Ludewig PM, Behrens SA, Meyer SM, Spoden SM, Wilson LA. Three-dimensional clavicular motion during arm elevation: reliability and descriptive data. The Journal of orthopaedic and sports physical therapy 2004;34:140-9.
- ↑ Broca A, Hartmann H. Contribution à l’étude des luxations de l’épaule (luxations anciennes et luxations récidivantes). Bull Soc Anat 1890;4:416–423
- ↑ Warner JJ, Deng XH, Warren RF, Torzilli PA. Static capsuloligamentous restraints to superior-inferior translation of the glenohumeral joint. Am J Sports Med 1992;20:675–685
- ↑ Pouliart N, Gagey O. Simulated humeral avulsion of the glenohumeral ligaments: a new instability model. J Shoulder Elbow Surg 2006;15:728–735
- ↑ Bigliani LU, Pollock RG, Soslowsky LJ, Flatow EL, Pawluk RJ, Mow VC. Tensile properties of the inferior glenohumeral ligament. J Orthop Res 1992;10:187–197
- ↑ Bey MJ, Hunter SA, Kilambi N, Butler DL, Lindenfeld TN. Structural and mechanical properties of the glenohumeral joint posterior capsule. J Shoulder Elbow Surg 2005;14:201–206
- ↑ Gerber C, Terrier F, Ganz R. The Trillat procedure for recurrent anterior instability of the shoulder. J Bone Joint Surg Br 1988;70:130–134
- ↑ Walch G, Agostini JY, Levigne C, Nove-Josserand L. Recurrent anterior and multidirectional instability of the shoulder. Rev Chir Orthop Repar Appar Mot 1995;81:682–690
- ↑ Gagey OJ, Gagey N. The hyperabduction test. J Bone Joint Surg Br 2001;83:69–74
- ↑ Hovelius L, Rahme H. Primary anterior dislocation of the shoulder: long-term prognosis at the age of 40 years or younger. Knee Surg Sports Traumatol Arthrosc 2016;24:330–342
- ↑ Johnson SM, Robinson CM. Shoulder instability in patients with joint hyperlaxity. J Bone Joint Surg Am 2010;92:1545–1557
- ↑ Remia LF, Ravalin RV, Lemly KS, McGarry MH, Kvitne RS, Lee TQ. Biomechanical evaluation of multidirectional glenohumeral instability and repair. Clin Orthop Relat Res 2003;416:225–236
- ↑ Schneider DJ, Tibone JE, McGarry MH, Grossman MG, Veneziani S, Lee TQ. Biomechanical evaluation after five and ten millimeter anterior glenohumeral capsulorrhaphy using a novel shoulder model of increased laxity. J Shoulder Elbow Surg 2005;14:318–323
- ↑ 33.0 33.1 Farber AJ, ElAttrache NS, Tibone JE, McGarry MH, Lee TQ. Biomechanical analysis comparing a traditional superior-inferior arthroscopic rotator interval closure with a novel medial-lateral technique in a cadaveric multidirectional instability model. Am J Sports Med 2009;37:1178–1185
- ↑ Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med 2011;39:526–531
- ↑ Shafer BL, Mihata T, McGarry MH, Tibone JE, Lee TQ. Effects of capsular plication and rotator interval closure in simulated multidirectional shoulder instability. J Bone Joint Surg Am 2008;90:136–144
- ↑ McPherson EJ, Friedman RJ, An YH, Chokesi R, Dooley RL. Anthropometric study of normal glenohumeral relationships. J Shoulder Elbow Surg 1997;6:105–112
- ↑ Pagnani MJ, Deng XH, Warren RF, Torzilli PA, Altchek DW. Effect of lesions of the superior portion of the glenoid labrum on glenohumeral translation. J Bone Joint Surg Am 1995;77:1003–1010
- ↑ Lippitt S, Matsen F. Mechanisms of glenohumeral joint stability. Clin Orthop Relat Res 1993;291:20–28
- ↑ Hara H, Ito N, Iwasaki K. Strength of the glenoid labrum and adjacent shoulder capsule. J Shoulder Elbow Surg 1996;5:263–268
- ↑ Kumar VP, Balasubramaniam P. The role of atmospheric pressure in stabilising the shoulder: an experimental study. J Bone Joint Surg Br 1985;67:719–721
- ↑ Habermeyer P, Schuller U, Wiedemann E. The intra-articular pressure of the shoulder: an experimental study on the role of the glenoid labrum in stabilizing the joint. Arthroscopy 1992;8:166–172
- ↑ Warner JJ, Bowen MK, Deng X, Torzilli PA, Warren RF. Effect of joint compression on inferior stability of the glenohumeral joint. J Shoulder Elbow Surg 1999;8:31–36
- ↑ Itoi E. Pathophysiology and treatment of atraumatic instability of the shoulder. J Orthop Sci 2004;9:208–213
- ↑ 44.0 44.1 Yamamoto N, Muraki T, Sperling JW, Steinmann SP, Cofield RH, Itoi E, An KN. Stabilizing mechanism in bone-grafting of a large glenoid defect. J Bone Joint Surg Am 2010;92:2059–2066
- ↑ 45.0 45.1 45.2 45.3 Mehl J, Otto A, Imhoff FB, Murphy M, Dyrna F, Obopilwe E, Cote M, Lädermann A, Collin P, Beitzel K, Mazzocca AD. Dynamic anterior shoulder stabilization with the long head of the biceps tendon: a biomechanical study. Am J Sports Med 2019;47:1441–1450
- ↑ 46.0 46.1 Shin SJ, Koh YW, Bui C, Jeong WK, Akeda M, Cho NS, McGarry MH, Lee TQ. What is the critical value of glenoid bone loss at which soft tissue Bankart repair does not restore glenohumeral translation, restricts range of motion, and leads to abnormal humeral head position? Am J Sports Med 2016;44:2784–2791
- ↑ Montgomery WH, Jr, Wahl M, Hettrich C, Itoi E, Lippitt SB, Matsen FA., III Anteroinferior bone-grafting can restore stability in osseous glenoid defects. J Bone Joint Surg Am 2005;87:1972–1977
- ↑ Ghodadra N, Gupta A, Romeo AA, Bach Jr BR, Verma N, Shewman E, Goldstein J, Provencher MT. Normalization of glenohumeral articular contact pressures after Latarjet or iliac crest bone-grafting. J Bone Joint Surg Am 2010;92:1478–1489
- ↑ Petersen SA, Bernard JA, Langdale ER, Belkoff SM. Autologous distal clavicle versus autologous coracoid bone grafts for restoration of anterior-inferior glenoid bone loss: a biomechanical comparison. J Shoulder Elbow Surg 2016;25:960–966
- ↑ Young AA, Baba M, Neyton L, Godeneche A, Walch G. Coracoid graft dimensions after harvesting for the open Latarjet procedure. J Shoulder Elbow Surg 2013;22:485–488
- ↑ de Beer JF, Roberts C. Glenoid bone defects: open Latarjet with congruent arc modification. Orthop Clin North Am 2010;41:407–415
- ↑ Colegate-Stone TJ, van der Watt C, de Beer JF. Evaluation of functional outcomes and complications following modified Latarjet reconstruction in athletes with anterior shoulder instability. Shoulder Elbow 2015;7:168–173
- ↑ Bhatia S, Van Thiel GS, Gupta D, Ghodadra N, Cole BJ, Bach BR Jr, Shewman E, Wang VM, Romeo AA, Verma NN, Provencher MT. Comparison of glenohumeral contact pressures and contact areas after glenoid reconstruction with Latarjet or distal tibial osteochondral allografts. Am J Sports Med 2013;41:1900–1908
- ↑ Savoie FH, III, Holt MS, Field LD, Ramsey JR. Arthroscopic management of posterior instability: evolution of technique and results. Arthroscopy 2008;24:389–396
- ↑ Wellmann M, Blasig H, Bobrowitsch E, Kobbe P, Windhagen H, Petersen W, Bohnsack M. The biomechanical effect of specific labral and capsular lesions on posterior shoulder instability. Arch Orthop Trauma Surg 2011;131:421–427
- ↑ Fuchs B, Jost B, Gerber C. Posterior-inferior capsular shift for the treatment of recurrent, voluntary posterior subluxation of the shoulder. J Bone Joint Surg Am 2000;82:16–25
- ↑ Imhoff FB, Camenzind RS, Obopilwe E, Cote MP, Mehl J, Beitzel K, Imhoff AB, Mazzocca AD, Arciero RA, Dyrna FGE. Glenoid retroversion is an important factor for humeral head centration and the biomechanics of posterior shoulder stability. Knee Surg Sports Traumatol Arthrosc 2019;27:3952–3961
- ↑ Owens BD, Campbell SE, Cameron KL. Risk factors for posterior shoulder instability in young athletes. Am J Sports Med 2013;41:2645–2649
- ↑ Malgaigne J. Traité des fractures et des luxations. Paris: J.-B. Baillière, 1855
- ↑ Hill H, Sachs M. The grooved defect of the humeral head: a frequently unrecognized complication of dislocations of the shoulder joint. Radiology 1940;35:690–700
- ↑ Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair: apparent causes of failure and treatment. J Bone Joint Surg Am 1984;66:159–168
- ↑ Boileau P, Villalba M, Héry JY, Balg F, Ahrens P, Neyton L. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am 2006;88:1755–1763
- ↑ Sekiya JK, Wickwire AC, Stehle JH, Debski RE. Hill–Sachs defects and repair using osteoarticular allograft transplantation: biomechanical analysis using a joint compression model. Am J Sports Med 2009;37:2459–2466
- ↑ Sekiya JK, Jolly J, Debski RE. The effect of a Hill–Sachs defect on glenohumeral translations, in situ capsular forces, and bony contact forces. Am J Sports Med 2012;40:388–394
- ↑ Bakshi NK, Jolly JT, Debski RE, Sekiya JK. Does repair of a Hill–Sachs defect increase stability at the glenohumeral joint? Orthop J Sports Med 2016;4:2325967116645091
- ↑ Lazarides AL, Duchman KR, Ledbetter L, Riboh JC, Garrigues GE. Arthroscopic remplissage for anterior shoulder instability: a systematic review of clinical and biomechanical studies. Arthroscopy 2019;35:617–628
- ↑ Degen RM, Giles JW, Johnson JA, Athwal GS. Remplissage versus latarjet for engaging Hill–Sachs defects without substantial glenoid bone loss: a biomechanical comparison. Clin Orthop Relat Res 2014;472:2363–2371
- ↑ Argintar E, Heckmann N, Wang L, Tibone JE, Lee TQ. The biomechanical effect of shoulder remplissage combined with Bankart repair for the treatment of engaging Hill–Sachs lesions. Knee Surg Sports Traumatol Arthrosc 2016;24:585–592
- ↑ Lädermann A, Arrigoni P, Barth J, Narbona P, Hanypsiak B, Burkhart SS, Denard PJ. Is arthroscopic remplissage a tenodesis or capsulomyodesis? An anatomic study. Knee Surg Sports Traumatol Arthrosc 2016;24:573–577
- ↑ McLaughlin HL. Locked posterior subluxation of the shoulder: diagnosis and treatment. Surg Clin North Am 1963;43:1621–1622
- ↑ Krackhardt T, Schewe B, Albrecht D, Weise K. Arthroscopic fixation of the subscapularis tendon in the reverse Hill–Sachs lesion for traumatic unidirectional posterior dislocation of the shoulder. Arthroscopy 2006;22:227.e1–227.e6
- ↑ Delcogliano A, Caporaso A, Chiossi S, Menghi A, Cillo M, Delcogliano M. Surgical management of chronic, unreduced posterior dislocation of the shoulder. Knee Surg Sports Traumatol Arthrosc 2005;13:151–155
- ↑ Banerjee M, Balke M, Bouillon B, Wafaisade A, Helm P, Akoto R, Shafizadeh S. Excellent results of lesser tuberosity transfer in acute locked posterior shoulder dislocation. Knee Surg Sports Traumatol Arthrosc 2013;21:2884–2888
- ↑ Yamamoto N, Itoi E, Abe H, Minagawa H, Seki N, Shimada Y, Okada K. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg 2007;16:649–656
- ↑ Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill–Sachs lesion. Arthroscopy 2000;16:677–694
- ↑ Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill–Sachs lesion: from ‘engaging/non-engaging’ lesion to ‘on-track/off-track’ lesion. Arthroscopy 2014;30:90–98
- ↑ Baudi P, Righi P, Bolognesi D, Rivetta S, Urtoler ER, Guicciardi N, Carrara M. How to identify and calculate glenoid bone deficit. Chir Organi Mov 2005;90:145–152
- ↑ Porcellini G, Caranzano F, Campi F, Pellegrini A, Paladini P. Glenohumeral instability and rotator cuff tear. Sports Med Arthrosc Rev 2011;19:395–400
- ↑ Labriola JE, Lee TQ, Debski RE, McMahon PJ. Stability and instability of the glenohumeral joint: the role of shoulder muscles. J Shoulder Elbow Surg 2005;14:32S–38S
- ↑ Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med 1994;22:121–130
- ↑ Pouliart N, Gagey O. Concomitant rotator cuff and capsuloligamentous lesions of the shoulder: a cadaver study. Arthroscopy 2006;22:728–735
- ↑ Rathi S, Taylor NF, Soo B, Green RA. Glenohumeral joint translation and muscle activity in patients with symptomatic rotator cuff pathology: an ultrasonographic and electromyographic study with age-matched controls. J Sci Med Sport 2018;21:885–889
- ↑ Itoi E, Newman SR, Kuechle DK, Morrey BF, An KN. Dynamic anterior stabilisers of the shoulder with the arm in abduction. J Bone Joint Surg Br 1994;76:834–836
- ↑ Kephart CJ, Abdulian MH, McGarry MH, Tibone JE, Lee TQ. Biomechanical analysis of the modified Bristow procedure for anterior shoulder instability: is the bone block necessary? J Shoulder Elbow Surg 2014;23:1792–1799
- ↑ Yamamoto N, Muraki T, An KN, et al. The stabilizing mechanism of the Latarjet procedure: a cadaveric study. J Bone Joint Surg Am 2013;95:1390–1397
- ↑ Giles JW, Degen RM, Johnson JA, Athwal GS. The Bristow and Latarjet procedures: why these techniques should not be considered synonymous. J Bone Joint Surg Am 2014;96:1340–1348
- ↑ Collin P, Lädermann A. Dynamic anterior stabilization using the long head of the biceps for anteroinferior glenohumeral instability. Arthrosc Tech 2017;7:e39–e44
- ↑ Barrett Payne W, Kleiner MT, McGarry MH, Tibone JE, Lee TQ. Biomechanical comparison of the Latarjet procedure with and without a coracoid bone block. Knee Surg Sports Traumatol Arthrosc 2016;24:513–520
- ↑ Bokshan SL, Gil JA, DeFroda SF, Badida R, Crisco JJ, Owens BD. Biomechanical comparison of the long head of the biceps tendon versus conjoint tendon transfer in a bone loss shoulder instability model. Orthop J Sports Med 2019;7:2325967119883549
- ↑ Meyer DC, Ernstbrunner L, Boyce G, Imam MA, El Nashar R, Gerber C. Posterior acromial morphology is significantly associated with posterior shoulder instability. J Bone Joint Surg Am 2019;101:1253–1260
- ↑ Lädermann A, Tirefort J, Zanchi D, Haller S, Charbonnier C, Hoffmeyer P, Cunningham G. Shoulder apprehension: a multifactorial approach. EFORT Open Rev 2018;3:550–557
- ↑ Charbonnier C, Lädermann A, Kevelham B, Chagué S, Hoffmeyer P, Holzer N. Shoulder strengthening exercises adapted to specific shoulder pathologies can be selected using new simulation techniques: a pilot study. Int J Comput Assist Radiol Surg 2018;13:321–330
- ↑ 93.0 93.1 Saha AK. The classic mechanism of shoulder movements and a plea for the recognition of ‘zero position’ of glenohumeral joint. Clin Orthop Relat Res 1983;173:3–10
- ↑ Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology Part I: pathoanatomy and biomechanics. Arthroscopy 2003;19:404–420
- ↑ Bagg SD, Forrest WJ. A biomechanical analysis of scapular rotation during arm abduction in the scapular plane. Am J Phys Med Rehabil 1988;67:238–245
- ↑ Black KP, Lim TH, McGrady LM, Raasch W. In vitro evaluation of shoulder external rotation after a Bankart reconstruction. Am J Sports Med 1997;25:449–453
- ↑ Penna J, Deramo D, Nelson CO, Sileo MJ, Levin SM, Tompkins B, Ianuzzi A.Determination of anterior labral repair stress during passive arm motion in a cadaveric model. Arthroscopy 2008;24:930–935
- ↑ Werner CM, Nyffeler RW, Jacob HA, Gerber C. The effect of capsular tightening on humeral head translations. J Orthop Res 2004;22:194–201
- ↑ 99.0 99.1 Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles: a cadaver study. J Bone Joint Surg Br 1993;75:137–140
- ↑ Collin P, Matsumura N, Lädermann A, Denard PJ, Walch G. Relationship between massive chronic rotator cuff tear pattern and loss of active shoulder range of motion. J Shoulder Elbow Surg 2014;23:1195–1202
- ↑ Nové-Josserand L, Edwards TB, O’Connor DP, Walch G. The acromiohumeral and coracohumeral intervals are abnormal in rotator cuff tears with muscular fatty degeneration. Clin Orthop Relat Res 2005;433:90–96
- ↑ Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s ‘suspension bridge’. Arthroscopy 1993;9:611–616
- ↑ 103.0 103.1 103.2 Huri G, Kaymakoglu M, Garbis N. Rotator cable and rotator interval: anatomy, biomechanics and clinical importance. EFORT Open Rev 2019;4:56–62.
- ↑ Mochizuki T, Sugaya H, Uomizu M, Maeda K, Matsuki K, Sekiya I, Muneta T, Akita K. Humeral insertion of the supraspinatus and infraspinatus: new anatomical findings regarding the footprint of the rotator cuff. Surgical technique. J Bone Joint Surg Am 2009;91:1–7
- ↑ Burkhart SS, Nottage WM, Ogilvie-Harris DJ, Kohn HS, Pachelli A. Partial repair of irreparable rotator cuff tears. Arthroscopy 1994;10:363–370
- ↑ Denard PJ, Koo SS, Murena L, Burkhart SS. Pseudoparalysis: the importance of rotator cable integrity. Orthopedics 2012;35:e1353–e1357
- ↑ Bouaicha S, Ernstbrunner L, Jud L, Meyer DC, Snedeker JG, Bachmann E. The lever arm ratio of the rotator cuff to deltoid muscle explains and predicts pseudoparalysis of the shoulder: the Shoulder Abduction Moment index. Bone Joint J 2018;100-B:1600–1608
- ↑ Leschinger T, Birgel S, Hackl M, Staat M, Müller LP, Wegmann K. A musculoskeletal shoulder simulation of moment arms and joint reaction forces after medialization of the supraspinatus footprint in rotator cuff repair. Comput Methods Biomech Biomed Engin 2019;22:595–604
- ↑ Liu J, Hughes RE, O’Driscoll SW, An KN. Biomechanical effect of medial advancement of the supraspinatus tendon: a study in cadavera. J Bone Joint Surg Am 1998;80:853–859
- ↑ Yamamoto N, Itoi E, Tuoheti Y, Seki N, Abe H, Minagawa H, Shimada Y, Okada K. Glenohumeral joint motion after medial shift of the attachment site of the supraspinatus tendon: a cadaveric study. J Shoulder Elbow Surg 2007;16:373–378
- ↑ Denard PJ, Burkhart SS. Medialization of the subscapularis footprint does not affect functional outcome of arthroscopic repair. Arthroscopy 2012;28:1608–1614
- ↑ Nimura A, Kato A, Yamaguchi K, Mochizuki T, Okawa A, Sugaya H, Akita K. The superior capsule of the shoulder joint complements the insertion of the rotator cuff. J Shoulder Elbow Surg 2012;21:867–872
- ↑ Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med 1997;25:609–613
- ↑ Gerber C, Zubler V, Hodler J, Catanzaro S, Jost B, Fucentese SF. Dynamic imaging and function of partial supraspinatus tendon tears. Arthroscopy 2011;27:1180–1186
- ↑ Pinkowsky GJ, ElAttrache NS, Peterson AB, Akeda M, McGarry MH, Lee TQ. Partial-thickness tears involving the rotator cable lead to abnormal glenohumeral kinematics. J Shoulder Elbow Surg 2017;26:1152–1158
- ↑ Mihata T, McGarry MH, Ishihara Y, Bui CN, Alavekios D, Neo M, Lee TQ. Biomechanical analysis of articular-sided partial-thickness rotator cuff tear and repair. Am J Sports Med 2015;43:439–446
- ↑ Yamamoto N, Muraki T, Sperling JW, Steinmann SP, Itoi E, Cofield RH, An KN. Contact between the coracoacromial arch and the rotator cuff tendons in nonpathologic situations: a cadaveric study. J Shoulder Elbow Surg 2010;19:681–687
- ↑ Gerber C, Snedeker JG, Baumgartner D, Viehöfer AF. Supraspinatus tendon load during abduction is dependent on the size of the critical shoulder angle: a biomechanical analysis. J Orthop Res 2014;32:952–957
- ↑ Gerber C, Catanzaro S, Betz M, Ernstbrunner L. Arthroscopic correction of the critical shoulder angle through lateral acromioplasty: a safe adjunct to rotator cuff repair. Arthroscopy 2018;34:771–780
- ↑ Denard PJ, Bahney TJ, Kirby SB, Orfaly RM. Contact pressure and glenohumeral translation following subacromial decompression: how much is enough? Orthopedics 2010;33:805
- ↑ Reddy A, Gulotta LV, Chen X, Castagna A, Dines DM, Warren RF, Kontaxis A. Biomechanics of lower trapezius and latissimus dorsi transfers in rotator cuff-deficient shoulders. J Shoulder Elbow Surg 2019;28:1257–1264
- ↑ Adams CR, DeMartino AM, Rego G, Denard PJ, Burkhart SS. The rotator cuff and the superior capsule: why we need both. Arthroscopy 2016;32:2628–2637
- ↑ Pauzenberger L, Heuberer PR, Dyrna F, Obopilwe E, Kriegleder B, Anderl W, Mazzocca AD. Double-layer rotator cuff repair: anatomic reconstruction of the superior capsule and rotator cuff improves biomechanical properties in repairs of delaminated rotator cuff tears. Am J Sports Med 2018;46:3165–3173
- ↑ Mihata T, Lee TQ, Watanabe C, Fukunishi K, Ohue M, Tsujimura T, Kinoshita M. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy 2013;29:459–470
- ↑ Denard PJ, Brady PC, Adams CR, Tokish JM, Burkhart SS. Preliminary results of arthroscopic superior capsule reconstruction with dermal allograft. Arthroscopy 2018;34:93–99
- ↑ Boutsiadis A, Chen S, Jiang C, Lenoir H, Delsol P, Barth J. Long head of the biceps as a suitable available local tissue autograft for superior capsular reconstruction: ‘the Chinese way’. Arthrosc Tech 2017;6:e1559–e1566
- ↑ Ishihara Y, Mihata T, Tamboli M, Nguyen L, Park KJ, McGarry MH, Takai S, Lee TQ. Role of the superior shoulder capsule in passive stability of the glenohumeral joint. J Shoulder Elbow Surg 2014;23:642–648
- ↑ Omid R, Stone MA, Lin CC, Patel NA, Itami Y, McGarry MH, Lee TQ. Biomechanical analysis of latissimus dorsi tendon transfer with and without superior capsule reconstruction using dermal allograft. J Shoulder Elbow Surg 2019;28:1523–1530
- ↑ Hast MW, Schmidt EC, Kelly JD, IV, Baxter JR. Computational optimization of graft tension in simulated superior capsule reconstructions. J Orthop Res 2018;36:2789–2796
- ↑ Altintas B, Scheidt M, Kremser V, Boykin R, Bhatia S, Sajadi KR, Mair S, Millett PJ. Superior Capsule Reconstruction for Irreparable Massive Rotator Cuff Tears: Does It Make Sense? A Systematic Review of Early Clinical Evidence. Am J Sports Med. 2020 Nov;48(13):3365-3375
- ↑ Lobao MH, Canham RB, Melvani RT, Abboud JA, Parks BG, Murthi AM. Biomechanics of biodegradable subacromial balloon spacer for irreparable superior rotator cuff tears: study of a cadaveric model. J Bone Joint Surg Am 2019;101:e49
- ↑ Chevalier Y, Pietschmann MF, Thorwächter C, Chechik O, Adar E, Dekel A, Müller PE. Biodegradable spacer reduces the subacromial pressure: a biomechanical cadaver study. Clin Biomech (Bristol, Avon) 2018;52:41–48
- ↑ 133.0 133.1 Omid R, Stone MA, Lin CC, Patel NA, Itami Y, McGarry MH, Lee TQ. Biomechanical analysis of anterior capsule reconstruction and latissimus dorsi transfer for irreparable subscapularis tears. J Shoulder Elbow Surg 2020;29:374–380
- ↑ Nelson GN, Namdari S, Galatz L, Keener JD. Pectoralis major tendon transfer for irreparable subscapularis tears. J Shoulder Elbow Surg 2014;23:909–918
- ↑ Konrad GG, Sudkamp NP, Kreuz PC, Jolly JT, McMahon PJ, Debski RE. Pectoralis major tendon transfers above or underneath the conjoint tendon in subscapularis-deficient shoulders: an in vitro biomechanical analysis. J Bone Joint Surg Am 2007;89:2477–2484
- ↑ Elhassan B, Christensen TJ, Wagner ER. Feasibility of latissimus and teres major transfer to reconstruct irreparable subscapularis tendon tear: an anatomic study. J Shoulder Elbow Surg 2014;23:492–499
- ↑ 137.0 137.1 Komperda KW, Adamson GJ, Itami Y, McGarry MH, Kantor A, Lin CC, Lee TQ. Anterior capsule reconstruction versus pectoralis major transfer for irreparable subscapularis tears involving the anterior capsule: a comparative biomechanical cadaveric study. Arthroscopy 2019;35:3002–3008
- ↑ Hand GC, Athanasou NA, Matthews T, Carr AJ. The pathology of frozen shoulder. J Bone Joint Surg Br. 2007;89(7):928-32
- ↑ Abrassart S, Kolo F, Piotton S, Chih-Hao Chiu J, Stirling P, Hoffmeyer P, Lädermann A. 'Frozen shoulder' is ill-defined. How can it be described better? EFORT Open Rev. 2020;5(5):273-279.
- ↑ 140.0 140.1 O'Brien SJ, Schwartz RS, Warren RF, Torzilli PA. Capsular restraints to anterior-posterior motion of the abducted shoulder: a biomechanical study. J Shoulder Elbow Surg. 1995;4(4):298-308
- ↑ Petchprapa CN, Beltran LS, Jazrawi LM, Kwon YW, Babb JS, Recht MP. The rotator interval: a review of anatomy, function, and normal and abnormal MRI appearance. AJR Am J Roentgenol. 2010;195(3):567-76
- ↑ Fayad F, Roby-Brami A, Yazbeck C, Hanneton S, Lefevre-Colau MM, Gautheron V, Poiraudeau S, Revel M. Three-dimensional scapular kinematics and scapulohumeral rhythm in patients with glenohumeral osteoarthritis or frozen shoulder. J Biomech. 2008;41(2):326-32
- ↑ 143.0 143.1 143.2 Hettrich CM, Gasinu S, Beamer BS, Stasiak M, Fox A, Birmingham P, Ying O, Deng XH, Rodeo SA. The effect of mechanical load on tendon-to-bone healing in a rat model. Am J Sports Med 2014;42:1233–1241
- ↑ imbel JA, Van Kleunen JP, Williams GR, Thomopoulos S, Soslowsky LJ. Long durations of immobilization in the rat result in enhanced mechanical properties of the healing supraspinatus tendon insertion site. J Biomech Eng 2007;129:400–404
- ↑ Mazuquin BF, Wright AC, Russell S, Monga P, Selfe J, Richards J. Effectiveness of early compared with conservative rehabilitation for patients having rotator cuff repair surgery: an overview of systematic reviews. Br J Sports Med 2018;52:111–121
- ↑ Tirefort J, Schwitzguebel AJ, Collin P, Nowak A, Plomb-Holmes C, Lädermann A. Postoperative mobilization after superior rotator cuff repair: sling versus no sling: a randomized prospective study. J Bone Joint Surg Am 2019;101:494–503
- ↑ Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg 2012;21:228–237
- ↑ Galatz LM, Charlton N, Das R, Kim HM, Havlioglu N, Thomopoulos S. Complete removal of load is detrimental to rotator cuff healing. J Shoulder Elbow Surg 2009;18:669–675
- ↑ Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng 2003;125:106–113
- ↑ Kibler WB, Ludewig PM, McClure PW, Michener LA, Bak K, Sciascia AD. Clinical implications of scapular dyskinesis in shoulder injury: the 2013 consensus statement from the ‘Scapular Summit’. Br J Sports Med 2013;47:877–885
- ↑ Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg 1998;7:599–605
- ↑ Sonnabend DH, Howlett CR, Young AA. Histological evaluation of repair of the rotator cuff in a primate model. J Bone Joint Surg Br 2010;92:586–594
- ↑ 153.0 153.1 McPherson EJ, Friedman RJ, An YH, Chokesi R, Dooley RL. Anthropometric study of normal glenohumeral relationships. J Shoulder Elbow Surg. 1997;6(2):105-12
- ↑ Lee SB, Kim KJ, O'Driscoll SW, Morrey BF, An KN. Dynamic glenohumeral stability provided by the rotator cuff muscles in the mid-range and end-range of motion. A study in cadavera. J Bone Joint Surg Am. 2000;82(6):849-57
- ↑ Bergmann G, Graichen F, Bender A, Rohlmann A, Halder A, Beier A, et al. In vivo gleno-humeral joint loads during forward flexion and abduction. Journal of biomechanics. 2011;44(8):1543-52
- ↑ 156.0 156.1 Knowles NK, Carroll MJ, Keener JD, Ferreira LM, Athwal GS. A comparison of normal and osteoarthritic humeral head size and morphology. J Shoulder Elbow Surg. 2016;25(3):502-9
- ↑ 157.0 157.1 157.2 Alolabi B, Youderian AR, Napolitano L, Szerlip BW, Evans PJ, Nowinski RJ, et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1740-6
- ↑ Buchler P, Farron A. Benefits of an anatomical reconstruction of the humeral head during shoulder arthroplasty: a finite element analysis. Clin Biomech (Bristol, Avon). 2004;19(1):16-23
- ↑ 159.0 159.1 Boileau P, Walch G. The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br. 1997;79(5):857-65
- ↑ Jun BJ, Iannotti JP, McGarry MH, Yoo JC, Quigley RJ, Lee TQ. The effects of prosthetic humeral head shape on glenohumeral joint kinematics: a comparison of non-spherical and spherical prosthetic heads to the native humeral head. J Shoulder Elbow Surg. 2013;22(10):1423-32
- ↑ Berghs BM, Derveaux T, Speeckaert W, Vanslambrouck K, De Wilde LF. Three-dimensional analysis of the orientation and the inclination of the rotator cuff footprint. J Shoulder Elbow Surg. 2011;20(4):637-45
- ↑ 162.0 162.1 Robertson DD, Yuan J, Bigliani LU, Flatow EL, Yamaguchi K. Three-dimensional analysis of the proximal part of the humerus: relevance to arthroplasty. J Bone Joint Surg Am. 2000;82(11):1594-602
- ↑ 163.0 163.1 163.2 163.3 163.4 Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500
- ↑ Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-8
- ↑ Getz CL, Ricchetti ET, Verborgt O, Brolin TJ. Normal and Pathoanatomy of the Arthritic Shoulder: Considerations for Shoulder Arthroplasty. J Am Acad Orthop Surg. 2019;27(24):e1068-e76
- ↑ Barth J, Garret J, Boutsiadis A, Sautier E, Geais L, Bothorel H, et al. Is global humeral head offset related to intramedullary canal width? A computer tomography morphometric study. J Exp Orthop. 2018;5(1):35
- ↑ Muench, L.N., Otto, A., Kia, C. et al. Rotational range of motion of elliptical and spherical heads in shoulder arthroplasty: a dynamic biomechanical evaluation. Arch Orthop Trauma Surg 2020 https://doi.org/10.1007/s00402-020-03587-0
- ↑ Terrier A, Ramondetti S, Merlini F, Pioletti DD, Farron A. Biomechanical consequences of humeral component malpositioning after anatomical total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(8):1184-90
- ↑ Youderian AR, Ricchetti ET, Drews M, Iannotti JP. Determination of humeral head size in anatomic shoulder replacement for glenohumeral osteoarthritis. J Shoulder Elbow Surg. 2014;23(7):955-63
- ↑ Favre P, Moor B, Snedeker JG, Gerber C. Influence of component positioning on impingement in conventional total shoulder arthroplasty. Clin Biomech (Bristol, Avon). 2008;23(2):175-83
- ↑ Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am. 2004;86(3):575-80
- ↑ Vaesel MT, Olsen BS, Sojbjerg JO, Helmig P, Sneppen O. Humeral head size in shoulder arthroplasty: a kinematic study. J Shoulder Elbow Surg. 1997;6(6):549-55
- ↑ Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-6
- ↑ 174.0 174.1 Keener JD, Chalmers PN, Yamaguchi K. The Humeral Implant in Shoulder Arthroplasty. J Am Acad Orthop Surg. 2017;25(6):427-38
- ↑ Hammond G, Tibone JE, McGarry MH, Jun BJ, Lee TQ. Biomechanical comparison of anatomic humeral head resurfacing and hemiarthroplasty in functional glenohumeral positions. J Bone Joint Surg Am. 2012;94(1):68-76
- ↑ Jeong J, Bryan J, Iannotti JP. Effect of a variable prosthetic neck-shaft angle and the surgical technique on replication of normal humeral anatomy. J Bone Joint Surg Am. 2009;91(8):1932-41
- ↑ Bryce CD, Davison AC, Okita N, Lewis GS, Sharkey NA, Armstrong AD. A biomechanical study of posterior glenoid bone loss and humeral head translation. J Shoulder Elbow Surg. 2010;19(7):994-1002
- ↑ 178.0 178.1 Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492-7
- ↑ Ovesen J, Nielsen S. Prosthesis position in shoulder arthroplasty. A cadaver study of the humeral component. Acta Orthop Scand. 1985;56(4):330-1
- ↑ Pearl ML, Volk AG. Retroversion of the proximal humerus in relationship to prosthetic replacement arthroplasty. J Shoulder Elbow Surg. 1995;4(4):286-9
- ↑ Doyle AJ, Burks RT. Comparison of humeral head retroversion with the humeral axis/biceps groove relationship: a study in live subjects and cadavers. J Shoulder Elbow Surg. 1998;7(5):453-7
- ↑ Itamura J, Dietrick T, Roidis N, Shean C, Chen F, Tibone J. Analysis of the bicipital groove as a landmark for humeral head replacement. J Shoulder Elbow Surg. 2002;11(4):322-6
- ↑ Raniga S, Knowles NK, West E, Ferreira LM, Athwal GS. The Walch type B humerus: glenoid retroversion is associated with torsional differences in the humerus. J Shoulder Elbow Surg. 2019;28(9):1801-8
- ↑ Hsu HC, Wu JJ, Chen TH, Lo WH, Yang DJ. The influence of abductor lever-arm changes after shoulder arthroplasty. J Shoulder Elbow Surg. 1993;2(3):134-40
- ↑ Bodrogi A, Athwal GS, Howard L, Zhang T, Lapner P. A reliable method of determining glenohumeral offset in anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28(8):1609-16
- ↑ Jacobson SR, Mallon WJ. The glenohumeral offset ratio: A radiographic study. J Shoulder Elbow Surg. 1993;2(3):141-6
- ↑ Harris TE, Jobe CM, Dai QG. Fixation of proximal humeral prostheses and rotational micromotion. J Shoulder Elbow Surg. 2000;9(3):205-10
- ↑ Raiss P, Schnetzke M, Wittmann T, Kilian CM, Edwards TB, Denard PJ, et al. Postoperative radiographic findings of an uncemented convertible short stem for anatomic and reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28(4):715-23
- ↑ Tross AK, Lädermann A, Wittmann T, Schnetzke M, Nolte PC, Collin P, et al. Subsidence of Uncemented Short Stems in Reverse Shoulder Arthroplasty-A Multicenter Study. J Clin Med. 2020;9(10)
- ↑ Lädermann A, Chiu JC, Cunningham G, Herve A, Piotton S, Bothorel H, et al. Do short stems influence the cervico-diaphyseal angle and the medullary filling after reverse shoulder arthroplasties? Orthop Traumatol Surg Res. 2020;106(2):241-6
- ↑ Terrier A, Goetti P, Becce F, Farron A. Reduction of scapulohumeral subluxation with posterior augmented glenoid implants in anatomic total shoulder arthroplasty: Short-term 3D comparison between pre- and post-operative CT. Orthop Traumatol Surg Res. 2020;106(4):681-6
- ↑ Kwon YW, Powell KA, Yum JK, Brems JJ, Iannotti JP. Use of three-dimensional computed tomography for the analysis of the glenoid anatomy. J Shoulder Elbow Surg. 2005;14(1):85-90
- ↑ Anglin C, Wyss UP, Pichora DR. Shoulder prosthesis subluxation: theory and experiment. J Shoulder Elbow Surg. 2000;9(2):104-14
- ↑ Franklin JL, Barrett WP, Jackins SE, Matsen FA, 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46
- ↑ Schoch B, Abboud J, Namdari S, Lazarus M. Glenohumeral Mismatch in Anatomic Total Shoulder Arthroplasty. JBJS Rev. 2017;5(9):e1
- ↑ Jun BJ, Lee TQ, McGarry MH, Quigley RJ, Shin SJ, Iannotti JP. The effects of prosthetic humeral head shape on glenohumeral joint kinematics during humeral axial rotation in total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25(7):1084-93
- ↑ Gauci MO, Deransart P, Chaoui J, Urvoy M, Athwal GS, Sanchez-Sotelo J, et al. Three-dimensional geometry of the normal shoulder: a software analysis. J Shoulder Elbow Surg. 2020;29(12):e468-e477
- ↑ Verhaegen F, Plessers K, Verborgt O, Scheys L, Debeer P. Can the contralateral scapula be used as a reliable template to reconstruct the eroded scapula during shoulder arthroplasty? J Shoulder Elbow Surg. 2018;27(6):1133-8
- ↑ Terrier A, Ston J, Larrea X, Farron A. Measurements of three-dimensional glenoid erosion when planning the prosthetic replacement of osteoarthritic shoulders. Bone Joint J. 2014;96-B(4):513-8
- ↑ De Wilde LF, Verstraeten T, Speeckaert W, Karelse A. Reliability of the glenoid plane. J Shoulder Elbow Surg. 2010;19(3):414-22
- ↑ 201.0 201.1 Moor BK, Bouaicha S, Rothenfluh DA, Sukthankar A, Gerber C. Is there an association between the individual anatomy of the scapula and the development of rotator cuff tears or osteoarthritis of the glenohumeral joint?: A radiological study of the critical shoulder angle. Bone Joint J. 2013;95-B(7):935-41
- ↑ Engelhardt C, Farron A, Becce F, Place N, Pioletti DP, Terrier A. Effects of glenoid inclination and acromion index on humeral head translation and glenoid articular cartilage strain. J Shoulder Elbow Surg. 2017;26(1):157-64
- ↑ Viehofer AF, Snedeker JG, Baumgartner D, Gerber C. Glenohumeral joint reaction forces increase with critical shoulder angles representative of osteoarthritis-A biomechanical analysis. J Orthop Res. 2016;34(6):1047-52
- ↑ Gerber C, Snedeker JG, Baumgartner D, Viehofer AF. Supraspinatus tendon load during abduction is dependent on the size of the critical shoulder angle: A biomechanical analysis. J Orthop Res. 2014;32(7):952-7
- ↑ 205.0 205.1 Watling JP, Sanchez JE, Heilbroner SP, Levine WN, Bigliani LU, Jobin CM. Glenoid component loosening associated with increased critical shoulder angle at midterm follow-up. J Shoulder Elbow Surg. 2018;27(3):449-54
- ↑ Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-60
- ↑ Bercik MJ, Kruse K, 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-6
- ↑ Walker KE, Simcock XC, Jun BJ, Iannotti JP, Ricchetti ET. Progression of Glenoid Morphology in Glenohumeral Osteoarthritis. J Bone Joint Surg Am. 2018;100(1):49-56
- ↑ Iannotti JP, Jun BJ, Patterson TE, Ricchetti ET. Quantitative Measurement of Osseous Pathology in Advanced Glenohumeral Osteoarthritis. J Bone Joint Surg Am. 2017;99(17):1460-8
- ↑ Domos P, Checchia CS, Walch G. Walch B0 glenoid: pre-osteoarthritic posterior subluxation of the humeral head. J Shoulder Elbow Surg. 2018;27(1):181-8
- ↑ Walch G, Ascani C, Boulahia A, Nove-Josserand L, Edwards TB. Static posterior subluxation of the humeral head: an unrecognized entity responsible for glenohumeral osteoarthritis in the young adult. J Shoulder Elbow Surg. 2002;11(4):309-14
- ↑ Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg. 2012;21(11):1526-33
- ↑ Sabesan VJ, Callanan M, Youderian A, Iannotti JP. 3D CT assessment of the relationship between humeral head alignment and glenoid retroversion in glenohumeral osteoarthritis. J Bone Joint Surg Am. 2014;96(8):e64
- ↑ Jacxsens M, Van Tongel A, Henninger HB, De Coninck B, Mueller AM, De Wilde L. A three-dimensional comparative study on the scapulohumeral relationship in normal and osteoarthritic shoulders. J Shoulder Elbow Surg. 2016;25(10):1607-15
- ↑ Beeler S, Hasler A, Gotschi T, Meyer DC, Gerber C. Different acromial roof morphology in concentric and eccentric osteoarthritis of the shoulder: a multiplane reconstruction analysis of 105 shoulder computed tomography scans. J Shoulder Elbow Surg. 2018;27(12):e357-e66
- ↑ Meyer DC, Riedo S, Eckers F, Carpeggiani G, Jentzsch T, Gerber C. Small anteroposterior inclination of the acromion is a predictor for posterior glenohumeral erosion (B2 or C). J Shoulder Elbow Surg. 2019;28(1):22-7
- ↑ Goetti P, Denard PJ, Collin P, Ibrahim M, Hoffmeyer P, Lädermann A. Shoulder biomechanics in normal and selected pathological conditions. EFORT Open Reviews. 2020;5(8):508-18
- ↑ Matsen F, Lippitt S. Shoulder surgery: principles and procedures. Matsen F, Lippitt S, editors. Philadelphia:: Saunders; 2003
- ↑ Lewis GS, Conaway WK, Wee H, Kim HM. Effects of anterior offsetting of humeral head component in posteriorly unstable total shoulder arthroplasty: Finite element modeling of cadaver specimens. J Biomech. 2017;53:78-83
- ↑ Kim HM, Chacon AC, Andrews SH, Roush EP, Cho E, Conaway WK, et al. Biomechanical benefits of anterior offsetting of humeral head component in posteriorly unstable total shoulder arthroplasty: A cadaveric study. J Orthop Res. 2016;34(4):666-74
- ↑ Terrier A, Larrea X, Malfroy Camine V, Pioletti DP, Farron A. Importance of the subscapularis muscle after total shoulder arthroplasty. Clin Biomech (Bristol, Avon). 2013;28(2):146-50
- ↑ Denard PJ, Noyes MP, Lädermann A. A Tensionable Method for Subscapularis Repair after Shoulder Arthroplasty. JSES Open Access. 2018;2(4):205-10
- ↑ Virk MS, Aiyash SS, Frank RM, Mellano CS, Shewman EF, Wang VM, Romeo AA. Biomechanical comparison of subscapularis peel and lesser tuberosity osteotomy for double-row subscapularis repair technique in a cadaveric arthroplasty model. J Orthop Surg Res. 2019;14(1):391
- ↑ Van Thiel GS, Wang VM, Wang FC, Nho SJ, Piasecki DP, Bach BR, Jr., Romeo AA. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(5):657-63
- ↑ Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Healing rates and subscapularis fatty infiltration after lesser tuberosity osteotomy versus subscapularis peel for exposure during shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(3):396-402
- ↑ Choate WS, Kwapisz A, Momaya AM, Hawkins RJ, Tokish JM. Outcomes for subscapularis management techniques in shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2018;27(2):363-70
- ↑ De Wilde LF, De Coninck T, De Neve F, Berghs BM. Subscapularis release in shoulder replacement determines structural muscular changes. Clin Orthop Relat Res. 2012;470(8):2193-201
- ↑ Miller BS, Joseph TA, Noonan TJ, Horan MP, Hawkins RJ. Rupture of the subscapularis tendon after shoulder arthroplasty: diagnosis, treatment, and outcome. J Shoulder Elbow Surg. 2005;14(5):492-6
- ↑ Shi LL, Jiang JJ, Ek ET, Higgins LD. Failure of the lesser tuberosity osteotomy after total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(2):203-9
- ↑ Werthel JD, Schoch BS, Hooke A, Sperling JW, An KN, Valenti P, Elhassan B. Biomechanical Effectiveness of Tendon Transfers to Restore Active Internal Rotation in Shoulder with Deficient Subscapularis with and without Reverse Shoulder Arthroplasty. J Shoulder Elbow Surg. 2021 May;30(5):1196-1206
- ↑ Nyffeler RW, Sheikh R, Atkinson TS, Jacob HA, Favre P, Gerber C. Effects of glenoid component version on humeral head displacement and joint reaction forces: an experimental study. J Shoulder Elbow Surg. 2006;15(5):625-9
- ↑ Shapiro TA, McGarry MH, Gupta R, Lee YS, Lee TQ. Biomechanical effects of glenoid retroversion in total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 Suppl):S90-5
- ↑ 233.0 233.1 Farron A, Terrier A, Buchler P. Risks of loosening of a prosthetic glenoid implanted in retroversion. J Shoulder Elbow Surg. 2006;15(4):521-6
- ↑ Abler D, Berger S, Terrier A, Becce F, Farron A, Buchler P. A statistical shape model to predict the premorbid glenoid cavity. J Shoulder Elbow Surg. 2018;27(10):1800-8
- ↑ Plessers K, Vanden Berghe P, Van Dijck C, Wirix-Speetjens R, Debeer P, Jonkers I, Vander Sloten J. Virtual reconstruction of glenoid bone defects using a statistical shape model. J Shoulder Elbow Surg. 2018;27(1):160-6
- ↑ Clavert P, Millett PJ, Warner JJ. Glenoid resurfacing: what are the limits to asymmetric reaming for posterior erosion? J Shoulder Elbow Surg. 2007;16(6):843-8
- ↑ Ghoraishian M, Abboud JA, Romeo AA, Williams GR, Namdari S. Augmented glenoid implants in anatomic total shoulder arthroplasty: review of available implants and current literature. J Shoulder Elbow Surg. 2019;28(2):387-95
- ↑ Ho JC, Amini MH, Entezari V, Jun BJ, Alolabi B, Ricchetti ET, Iannoti JP. Clinical and Radiographic Outcomes of a Posteriorly Augmented Glenoid Component in Anatomic Total Shoulder Arthroplasty for Primary Osteoarthritis with Posterior Glenoid Bone Loss. J Bone Joint Surg Am. 2018;100(22):1934-48
- ↑ Karelse A, Van Tongel A, Verstraeten T, Poncet D, De Wilde LF. Rocking-horse phenomenon of the glenoid component: the importance of inclination. J Shoulder Elbow Surg. 2015;24(7):1142-8
- ↑ Braun S, Schroeder S, Mueller U, Sonntag R, Buelhoff M, Kretzer JP. Influence of joint kinematics on polyethylene wear in anatomic shoulder joint arthroplasty. J Shoulder Elbow Surg. 2018;27(9):1679-85
- ↑ Mueller U, Braun S, Schroeder S, Schroeder M, Sonntag R, Jaeger S, Kretzer JP. Influence of humeral head material on wear performance in anatomic shoulder joint arthroplasty. J Shoulder Elbow Surg. 2017;26(10):1756-64
- ↑ Junaid S, Sanghavi S, Anglin C, Bull A, Emery R, Amis AA, Hansen Ul. Treatment of the Fixation Surface Improves Glenoid Prosthesis Longevity in vitro. Journal of biomechanics. 2017;61:81-7
- ↑ Gagliano JR, Helms SM, Colbath GP, Przestrzelski BT, Hawkins RJ, DesJardins JD. A comparison of onlay versus inlay glenoid component loosening in total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(7):1113-20
- ↑ Castagna A, Randelli M, Garofalo R, Maradei L, Giardella A, Borroni M. Mid-term results of a metal-backed glenoid component in total shoulder replacement. J Bone Joint Surg Br. 2010;92(10):1410-5
- ↑ Castagna A, Garofalo R. Journey of the glenoid in anatomic total shoulder replacement. Shoulder Elbow. 2019;11(2):140-8
- ↑ Somerson JS, Sander P, Bohsali K, Tibbetts R, Rockwood CA, Jr., Wirth MA. What Factors are Associated With Clinically Important Improvement After Shoulder Hemiarthroplasty for Cuff Tear Arthropathy? Clin Orthop Relat Res. 2016;474(12):2682-8
- ↑ Mahony GT, Werner BC, Chang B, Grawe BM, Taylor SA, Craig EV, et al. Risk factors for failing to achieve improvement after anatomic total shoulder arthroplasty for glenohumeral osteoarthritis. J Shoulder Elbow Surg. 2018;27(6):968-75
- ↑ Grammont PM, Trouilloud P, Latfay J, Deries X. Etude et réalisation d’une nouvelle prothèse d’épaule. Rhumatologie. 1987;39:407-18
- ↑ Middernacht B, Van Tongel A, De Wilde L. A Critical Review on Prosthetic Features Available for Reversed Total Shoulder Arthroplasty. Biomed Res Int. 2016;2016:3256931
- ↑ 250.0 250.1 Kontaxis A, Johnson GR. The biomechanics of reverse anatomy shoulder replacement--a modelling study. Clin Biomech (Bristol, Avon). 2009;24(3):254-60
- ↑ 251.0 251.1 Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 Suppl S):147S-61S
- ↑ Schwartz DG, Kang SH, Lynch TS, Edwards S, Nuber G, Zhang LQ, et al. The anterior deltoid's importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-64
- ↑ Lädermann A, Walch G, Denard PJ, Collin P, Sirveaux F, Favard L, et al. Reverse shoulder arthroplasty in patients with pre-operative impairment of the deltoid muscle. Bone Joint J. 2013;95-B(8):1106-13
- ↑ Gulotta LV, Choi D, Marinello P, Wright T, Cordasco FA, Craig EV, et al. Anterior deltoid deficiency in reverse total shoulder replacement: a biomechanical study with cadavers. J Bone Joint Surg Br. 2012;94(12):1666-9
- ↑ Ackland DC, Richardson M, Pandy MG. Axial rotation moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2012;94(20):1886-95
- ↑ Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1221-30
- ↑ Herrmann S, Konig C, Heller M, Perka C, Greiner S. Reverse shoulder arthroplasty leads to significant biomechanical changes in the remaining rotator cuff. J Orthop Surg Res. 2011;6:4
- ↑ Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2007;89(5):934-9
- ↑ Shi LL, Cahill KE, Ek ET, Tompson JD, Higgins LD, Warner JJ. Latissimus Dorsi and Teres Major Transfer With Reverse Shoulder Arthroplasty Restores Active Motion and Reduces Pain for Posterosuperior Cuff Dysfunction. Clin Orthop Relat Res. 2015;473(10):3212-7
- ↑ Greiner S, Schmidt C, Konig C, Perka C, Herrmann S. Lateralized reverse shoulder arthroplasty maintains rotational function of the remaining rotator cuff. Clin Orthop Relat Res. 2013;471(3):940-6
- ↑ Kwon YW, Pinto VJ, Yoon J, Frankle MA, Dunning PE, Sheikhzadeh A. Kinematic analysis of dynamic shoulder motion in patients with reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(9):1184-90
- ↑ 262.0 262.1 262.2 Harman M, Frankle M, Vasey M, Banks S. Initial glenoid component fixation in "reverse" total shoulder arthroplasty: a biomechanical evaluation. J Shoulder Elbow Surg. 2005;14(1 Suppl S):162S-7S
- ↑ Terrier A, Reist A, Merlini F, Farron A. Simulated joint and muscle forces in reversed and anatomic shoulder prostheses. J Bone Joint Surg Br. 2008;90(6):751-6
- ↑ Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Muscle and joint-contact loading at the glenohumeral joint after reverse total shoulder arthroplasty. J Orthop Res. 2011;29(12):1850-8
- ↑ Rugg CM, Coughlan MJ, Lansdown DA. Reverse Total Shoulder Arthroplasty: Biomechanics and Indications. Curr Rev Musculoskelet Med. 2019;12(4):542-53
- ↑ Lädermann A, Gueorguiev B, Charbonnier C, Stimec BV, Fasel JH, Zderic I, et al. Scapular Notching on Kinematic Simulated Range of Motion After Reverse Shoulder Arthroplasty Is Not the Result of Impingement in Adduction. Medicine (Baltimore). 2015;94(38):e1615
- ↑ Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89(3):588-600
- ↑ 268.0 268.1 Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-8
- ↑ 269.0 269.1 de Wilde LF, Poncet D, Middernacht B, Ekelund A. Prosthetic overhang is the most effective way to prevent scapular conflict in a reverse total shoulder prosthesis. Acta Orthop. 2010;81(6):719-26
- ↑ Lädermann A, Denard PJ, Collin P, Zbinden O, Chiu JC, Boileau P, et al. Effect of humeral stem and glenosphere designs on range of motion and muscle length in reverse shoulder arthroplasty. Int Orthop. 2020;44(3):519-30
- ↑ Haidamous G, Lädermann A, Hartzler RU, Parsons B, Lederman E, Tokish J, et al. Radiographic parameters associated with excellent versus poor range of motion outcomes following reverse shoulder arthroplasty. Shoulder & Elbow. 2020;9:1758573220936234
- ↑ Roche C, Flurin PH, Wright T, Crosby LA, Mauldin M, Zuckerman JD. An evaluation of the relationships between reverse shoulder design parameters and range of motion, impingement, and stability. J Shoulder Elbow Surg. 2009;18(5):734-41
- ↑ 273.0 273.1 Gutierrez S, Comiskey CAt, Luo ZP, Pupello DR, Frankle MA. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. Hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am. 2008;90(12):2606-15
- ↑ Lädermann A, Denard PJ, Boileau P, Farron A, Deransart P, Terrier A, et al. Effect of humeral stem design on humeral position and range of motion in reverse shoulder arthroplasty. Int Orthop. 2015;39(11):2205-13
- ↑ Gutierrez S, Levy JC, Frankle MA, Cuff D, Keller TS, Pupello DR, et al. Evaluation of abduction range of motion and avoidance of inferior scapular impingement in a reverse shoulder model. J Shoulder Elbow Surg. 2008;17(4):608-15 Gutierrez S, Levy JC, Frankle MA, Cuff D, Keller TS, Pupello DR, et al. Evaluation of abduction range of motion and avoidance of inferior scapular impingement in a reverse shoulder model. J Shoulder Elbow Surg. 2008;17(4):608-15
- ↑ Kim SJ, Jang SW, Jung KH, Kim YS, Lee SJ, Yoo YS. Analysis of impingement-free range of motion of the glenohumeral joint after reverse total shoulder arthroplasty using three different implant models. J Orthop Sci. 2019;24(1):87-94
- ↑ 277.0 277.1 Lädermann A, Tay E, Collin P, Piotton S, Chiu CH, Michelet A, et al. Effect of critical shoulder angle, glenoid lateralization, and humeral inclination on range of movement in reverse shoulder arthroplasty. Bone Joint Res. 2019;8(8):378-86
- ↑ Wong MT, Langohr GDG, Athwal GS, Johnson JA. Implant positioning in reverse shoulder arthroplasty has an impact on acromial stresses. J Shoulder Elbow Surg. 2016;25(11):1889-95
- ↑ 279.0 279.1 Haidamous G, Lädermann A, Frankle MA, Gorman RA, 2nd, Denard PJ. The risk of postoperative scapular spine fracture following reverse shoulder arthroplasty is increased with an onlay humeral stem. J Shoulder Elbow Surg. 2020;29(12):2556-63
- ↑ 280.0 280.1 Berliner JL, Regalado-Magdos A, Ma CB, Feeley BT. Biomechanics of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(1):150-60
- ↑ Lädermann A, Schwitzguebel AJ, Edwards TB, Godeneche A, Favard L, Walch G, et al. Glenoid loosening and migration in reverse shoulder arthroplasty. Bone Joint J. 2019;101-B(4):461-9
- ↑ 282.0 282.1 Giles JW, Langohr GD, Johnson JA, Athwal GS. Implant Design Variations in Reverse Total Shoulder Arthroplasty Influence the Required Deltoid Force and Resultant Joint Load. Clin Orthop Relat Res. 2015;473(11):3615-26
- ↑ Boileau P, Moineau G, Roussanne Y, O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-67
- ↑ Jasty M, Bragdon C, Burke D, O'Connor D, Lowenstein J, Harris WH. In vivo skeletal responses to porous-surfaced implants subjected to small induced motions. J Bone Joint Surg Am. 1997;79(5):707-14
- ↑ Hopkins AR, Hansen UN, Bull AM, Emery R, Amis AA. Fixation of the reversed shoulder prosthesis. J Shoulder Elbow Surg. 2008;17(6):974-80
- ↑ Chebli C, Huber P, Watling J, Bertelsen A, Bicknell RT, Matsen F, 3rd. Factors affecting fixation of the glenoid component of a reverse total shoulder prothesis. J Shoulder Elbow Surg. 2008;17(2):323-7
- ↑ James J, Allison MA, Werner FW, McBride DE, Basu NN, Sutton LG, et al. Reverse shoulder arthroplasty glenoid fixation: is there a benefit in using four instead of two screws? J Shoulder Elbow Surg. 2013;22(8):1030-6
- ↑ Bonnevialle N, Geais L, Muller JH, Shoulder Friends I, Berhouet J. Effect of RSA glenoid baseplate central fixation on micromotion and bone stress. JSES Int. 2020;4(4):979-86
- ↑ 289.0 289.1 289.2 Gutierrez S, Keller TS, Levy JC, Lee WE, 3rd, Luo ZP. Hierarchy of stability factors in reverse shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):670-6
- ↑ Gutierrez S, Walker M, Willis M, Pupello DR, Frankle MA. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(5):732-9
- ↑ Boileau P, Gauci MO, Wagner ER, Clowez G, Chaoui J, Chelli M, et al. The reverse shoulder arthroplasty angle: a new measurement of glenoid inclination for reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28(7):1281-90
- ↑ Walker M, Brooks J, Willis M, Frankle M. How reverse shoulder arthroplasty works. Clin Orthop Relat Res. 2011;469(9):2440-51
- ↑ 293.0 293.1 293.2 Clouthier AL, Hetzler MA, Fedorak G, Bryant JT, Deluzio KJ, Bicknell RT. Factors affecting the stability of reverse shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2013;22(4):439-44
- ↑ 294.0 294.1 294.2 Chae J, Siljander M, Wiater JM. Instability in Reverse Total Shoulder Arthroplasty. J Am Acad Orthop Surg. 2018;26(17):587-96
- ↑ Ackland DC, Robinson DL, Wilkosz A, Wu W, Richardson M, Lee P, Tse KM. The influence of rotator cuff tears on muscle and joint-contact loading after reverse total shoulder arthroplasty. J Orthop Res. 2019;37(1):211-9
- ↑ 296.0 296.1 296.2 Pastor MF, Kraemer M, Wellmann M, Hurschler C, Smith T. Anterior stability of the reverse shoulder arthroplasty depending on implant configuration and rotator cuff condition. Arch Orthop Trauma Surg. 2016;136(11):1513-9
- ↑ Langohr GD, Giles JW, Athwal GS, Johnson JA. The effect of glenosphere diameter in reverse shoulder arthroplasty on muscle force, joint load, and range of motion. J Shoulder Elbow Surg. 2015;24(6):972-9
- ↑ Gutierrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE, 3rd. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg. 2007;16(3 Suppl):S9-S12
- ↑ 299.0 299.1 Ferle M, Pastor MF, Hagenah J, Hurschler C, Smith T. Effect of the humeral neck-shaft angle and glenosphere lateralization on stability of reverse shoulder arthroplasty: a cadaveric study. J Shoulder Elbow Surg. 2019;28(5):966-73
- ↑ Henninger HB, Barg A, Anderson AE, Bachus KN, Burks RT, Tashjian RZ. Effect of lateral offset center of rotation in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2012;21(9):1128-35
- ↑ Favre P, Sussmann PS, Gerber C. The effect of component positioning on intrinsic stability of the reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(4):550-6
- ↑ Abdulla I, Langohr DG, Giles JW, Johnson JA, Athwal GS. The effect of humeral polyethylene insert constraint on reverse shoulder arthroplasty biomechanics. Shoulder Elbow. 2018;10(1):25-31
- ↑ 303.0 303.1 Lädermann A, Williams MD, Melis B, Hoffmeyer P, Walch G. Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):588-95
- ↑ Lädermann A, Walch G, Lubbeke A, Drake GN, Melis B, Bacle G, et al. Influence of arm lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(3):336-41
- ↑ Athwal GS, MacDermid JC, Reddy KM, Marsh JP, Faber KJ, Drosdowech D. Does bony increased-offset reverse shoulder arthroplasty decrease scapular notching? J Shoulder Elbow Surg. 2015;24(3):468-73
- ↑ Tashjian RZ, Burks RT, Zhang Y, Henninger HB. Reverse total shoulder arthroplasty: a biomechanical evaluation of humeral and glenosphere hardware configuration. J Shoulder Elbow Surg. 2015;24(3):e68-77
- ↑ Pegreffi F, Pellegrini A, Paladini P, Merolla G, Belli G, Velarde PU, Porcellini G. Deltoid muscle activity in patients with reverse shoulder prosthesis at 2-year follow-up. Musculoskelet Surg. 2017;101(Suppl 2):129-35
- ↑ Liou W, Yang Y, Petersen-Fitts GR, Lombardo DJ, Stine S, Sabesan VJ. Effect of lateralized design on muscle and joint reaction forces for reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(4):564-72
- ↑ Hamilton MA, Diep P, Roche C, Flurin PH, Wright TW, Zuckerman JD, et al. Effect of reverse shoulder design philosophy on muscle moment arms. J Orthop Res. 2015;33(4):605-13