Partial-thickness Posterosuperior Rotator Cuff Tears
[[Category:Shoulder]]
Contents
Bullet points
- Partial-thickness rotator cuff tears present partial disruption of tendon fibers with no communication between the subacromial bursa and the glenohumeral joint.
- The clinical presentation is surprisingly variable, ranging from mild discomfort to chronic pain, decreased throwing velocity, and shoulder disability.
- Partial tears are difficult to diagnose. Magnetic resonance arthrogram (MRA) is the best modality for radiographic assessment.
- The first approach to a partial-thickness rotator cuff tears is usually conservative, even if mechanical factors often result in poor spontaneous tendon healing.
- The initial approach is usually conservative. Platelet-Rich Plasma (PRP) injections do seem to result in clinical and radiological improvement.
- Surgical treatment: No agreement has been reached on the best surgical treatment, which include: rotator cuff debridement, rotator cuff or labral repair, glenoplasty, and subacromial decompression if necessary.
Key words
Shoulder arthroscopy; Partial-thickness rotator cuff tear; Delaminated; Nondelaminated; Debridement; Transtendon repair; Conversion to full-thickness tear; Take-down; En masse.
Introduction
Partial-thickness rotator cuff tears, characterised by disruption of tendon fibers with no communication between the subacromial bursa and the glenohumeral joint, are known causes of shoulder pain and disability.[1]
Described by Codman et al., these generally include the supraspinatus and infraspinatus, whereas partial-thickness subscapularis tears have a different pathogenesis and will not be analysed in this section (complete lesion).[2][3][4]
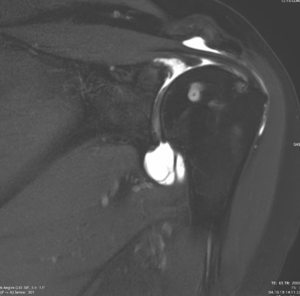
They may be on the bursal side (or superficial), interstitial only, or located in the deep surface adjacent to the joint (articular) (Figure 1), and are sometimes called partial articular supraspinatus tendon avulsion (PASTA) lesions.[2]
Partial-thickness rotator cuff tears are common, affecting approximately 5% to 20% of patients who present shoulder pain.[5][6]
The prevalence varies according to the type; 18% have a bursal component, 55% are interstitial and 27% are articular.[7]
Kim et al. monitored partial-thickness lesions with iterative Magnetic Resonance Images (MRI). At an average follow-up of 2 years, 26.1% of cases showed tear progression, while a decrease in size was observed in only 6.8% of cases.[8]
In another study involving more than half of the tendon insertion, at an average follow-up of 20 months, Kong et al. found that the tears had worsened in 16%, improved in 25%, and remained stable in 59% of cases.[9]
Classification
While several classifications have been proposed, there is no consensus as to general treatment.[10]
We therefore confine ourselves to the most used; that of Ellman et al., which divides the thickness of the rotator cuff into three more or less equal parts, varying between 10mm and 12mm.
Tears <3mm thick are termed stage I, those between 3 and 6 mm thick, stage II, and those affecting more than 50% of tendon thickness, stage III.[1]
Anatomy and biomechanics
Because the location of the tear is closely linked to its etiology, a good understanding of the anatomy and biomechanics is crucial. The insertion area of the supraspinatus tendon has recently been re-examined.[11]
It is a small triangular area measuring 6.9 mm in the coronal plane and 12.6 mm in the sagittal plane, extending to the lesser tubercle in 21% of cases. The infraspinatus insertion had previously been underestimated. It is trapezoidal in shape, covering a large part of the greater tuberostiy, 10.2 mm wide in the coronal plane and 32.7 mm long in the sagittal plane. Near the insertion, the two tendons are superimposed, the infraspinatus enveloping the supraspinatus. These different layers have variable rigidities which account for delamination.[12]
A study by Pouliart et al. found a superior joint capsule in 27.4% of cases. The complex entanglement of the different fibres from the infraspinatus, supraspinatus and possibly the joint capsule accounts for the different layers and the variability of lesions found within them (Figure 2).[13]

Partial-thickness tears have been for long wrongly considered to have little or no influence on shoulder kinematics. However, Gerber et al. have shown that a partial-thickness tear can have the same biomechanical repercussions as a full-thickness tear.[14]
Moreover, Pinkowsky et al. have recently confirmed, in a cadaveric study, that stage III tears affecting the rotator cable can lead to increased glenohumeral translation and altered kinematics.[15]
Studies assessing rotator cuff vascularization are inconsistent.[16]
Codman et al. describe hypovascularization in an area 5 mm to 10 mm from the rotator cuff insertion.[2]
This hypovascularization of the torn tendon area and the continuous mechanical stresses to which the rotator cuff is subjected, could be responsible for poor spontaneous healing following a partial-thickness tear. Conversely, in other more recent, notably cadaveric ones, the entire tendinous and bursal insertion appears uniformly vascularized.[17]
Etiology
Apart from the usual biological causes of rotator cuff tendinopathies (hyperlipidaemia, smoking, obesity, diabetes, hypertension, alcohol, fluoroquinolones, anti-inflammatories, thyroid disorders), partial damage to the cuff may be due to internal or external impingement, and intrinsic or extrinsic causes.
It should be emphasised that it is often difficult to precisely attribute the origin of the tear; the etiologies are multifactorial, resulting from a combination of negative factors.[18]
Bursal tears
Bursal-side tears are typically associated with external impingement (subacromial) or trauma.[19]
Interstitial Tears
Interstitial tears may be the result of repeated microtraumatic injuries, in the context of a mechanical phenomenon related to shear stresses between the different layers, as happens during throwing activities.
Articular tears
Articular-sided tears (Figure 3) usually occur in throwing athletes and racquet sports players. Thanks to a precise kinematic analysis, the posterosuperior internal impingement syndrome described by Walch et al.[20] has been dismantled.[21] The original cause is repeated friction during activities when the arm is hyperabducted in external rotation, but not, as previously suggested by other authors, joint movement limitation in internal rotation or subtle posterosuperior static instability or anteroinferior dynamics.[21]
A new theory has emerged in recent years. It appears that a significant lateral offset of the acromion, reflected by the critical shoulder angle (Figure 4), can cause an intrinsic mechanical overload to the cuff’s deep surface during arm movements at approximately 30° of abduction.[22]

Lastly, joint injury should also be suspected in patients who remain symptomatic after a slightly displaced fracture of the lesser tubercle.[23]
Diagnosis
The clinical presentation is surprisingly variable, ranging from mild discomfort to chronic pain or decreased throwing velocity. There is no clinical test to differentiate between partial-thckness and full-thickness tears.
The different tests for the posterosuperior rotator cuff (Jobe's maneuver, opposed external rotation strength with elbows at the side - which is the most accurate), are useful but painful.[24][25][26]
Moreover, arthroscopy provides no definitive diagnosis, as it does not reveal interstitial tearing. Only imaging can refine the diagnosis. Ultrasound (US) is an accurate method for assessing partial-thickness tears (Figures 5, 6), but as usual, the examiner's experience and specialization are crucial.[27]


Computed tomography (CT) arthrography should be outlawed, in view of the high radiation and the impossibility of detecting bursal and interstitial tears. Magnetic resonance imaging (MRI) provides additional information but does not specify the interstitial nature of the tear. It has a sensitivity of 51.6%, specificity of 77.2% with a positive predictive value of 41.3% and a negative predictive value of 83.7%.[26]
Only Magnetic resonance arhtrography (MRA) provides an accurate and complete diagnosis: fat-saturated T2 sequence gives a signal in the tear identical to the intra-articular signal, while fat-saturated T1 sequences signal the absence of gadolinium uptake in the subacromial bursa.[30][31]
Treatment
Conservative treatment
Conservative treatment is usually the best option.[32]
This applies to partial-thickness tears causing only moderate functional limitation and when the repair is difficult, sometimes requiring completion along the full thickness of the tendon, which, in 10% of the cases fail to heal.[33]
The duration of conservative treatment depends on the clinical presentation, physical and imaging observations, the degree of disability and the patient's demands and expectations. Bursal-sided tears in young patients who respond poorly to conservative treatment will be quickly operated. Similarly, an articular-sided tear with retraction of the deep layer, which could result in a very thin and insufficient rotator cuff, will be swiftly treated (Figure 7). Conversely, conservative treatment should be maximised when there is an extensive articular tear in the context of posterosuperior impingement in sports professionals wishing to remain active, and in cases of Ellman I-II type interstitial tears having no substantial extension into the musculotendinous junction.

Preferred conservative strategies therefore depend on the type of tear. In general, the patient will be asked to bide his/her time, to rest his/her injury by taking-up some other activity, make use of hot and cold compresses, and take non-steroidal anti-inflammatory drugs. Gentle exercises maintaining and increasing range of motion are recommended, as is a physiotherapy program designed to 1) maintain and/or improve normal scapulohumeral and scapulothoracic kinematics, by stretching of the posterior capsule, recentering the humeral head and flexing the periscapular muscles, especially the pectoralis minor, 2) to rebalance muscular strength around the shoulder in order to sustain the corrections (flexing the adductors and internal rotators, overused by throwing, strengthening the external cuff rotators, serratus anterior, trapezius inferior) and 3) to restore the cuff’s compressive strength and a good scapulohumeral/scapulothoracic synergy using neuromotor rehabilitation.
Adjuvent treatments must then be guided by the type of tear. Subacromial infiltrations (up to 3 injections) of steroidal derivatives for bursal tears, or intra-articular injections for joint tears, may well be useful at the outset. Platelet-Rich Plasma (PRP) injections are ineffective in cases of bursal and joint tears, because the ingredient is inevitably diluted in the subacromial and articular space, respectively. Prospective comparative study demonstrated that Platelet-Rich Plasma (PRP) has also no effect on the healing of interstitial rotator cuff tears, given that there are virtually no other indications for the use of Platelet-Rich Plasma (PRP) in the shoulder.[34]
Surgical treatment
The lack of concrete scientific data excludes precise guidelines for a correct surgical approach. Treatment protocols are generally pragmatic, and sometimes empirical. Usually the choice is made during arthroscopy, allowing for the age of the patient, their activities - especially among throwing athletes - the location and estimated depth of the tear, tissue quality, and the surgeon's experience. Surgical options for partial-thickness rotator cuff tears include debridement, cuff and labum repair, glenoplasty and subacromial decompression. It was previously agreed that surgical repair should be only be performed when more than 50% of the the tendon thickness was affected.41 However, indication should depend more on the conditions presented; the repair must be systematic for certain indications and completely contraindicated for others.
Bursal tears
The literature shows that, if operated, bursal tears warrant repair, regardless of size.[35]
The tear is completed if more than 50% of the tendon thickness is affected (Figure 8). In a retrospective study of 59 patients, Xiao et al. observed a 65 to 94 point improvement in the Constant score and a healing rate of 83.7% (N = 41).[36]
Acromioplasty is performed if the origin is degenerative.

Interstitial tears
A transtendinous repair or removal of the bursal layer, and cuff repair using an external brace, taking care to also pass the wires through the deep layer, is possible. In case of delamination extending to the musculotendinous junction, suturing between the two superoinferior layers may be included (Figure 9). There is no evidence that acromioplasty is effective for this type of tear.[37]

Articular tears in non-overhead throwers
Ellman I or II joint tears will simply be debrided. Ellman III tears will either be completed or repaired, conserving the bursal side (transtendinous repair). There is no evidence to prefer either technique.[38]
Nevertheless, incompleted repairs are biomechanically stronger and avoid the risk of non-healing of the bursal layer.[39][40][33]
It should be noted, however, that this type of repair leads to a 10% short term incidence of frozen shoulder.[38]
For all these mainly articular tears, where the critical shoulder angle (CSA) exceeds 33 degrees, a lateral acromioplasty might be justified in order to reduce the stress on the supraspinatus by limiting overload (intrinsic factor).[41]
Joint tears in throwing athletes with posterosuperior impingement
These have high prevalence but are usually asymptomatic, and should be treated conservatively for as long as possible. Moreover, they do not necessarily progress, and therefore simple observation is recommended.
While partial thickness tear repair is effective, the benefit to throwers is still uncertain. In this group, the therapeutic approach has recently changed, insofar that previous hypotheses regarding dynamic anterior instability, static posterosuperior instability, rotational disorders, etc. are still unconfirmed.
The cuff should not be repaired as it may cause shortening of the muscle-tendon, anatomic and mechanical changes to the shoulder and loss of range, preventing the athlete from throwing. Therefore, if surgery is unavoidable, a glenoplasty with resection or labral repair, depending on the state of the labrum could be proposed.[42]
Acromioplasty is not indicated for this etiology.
Postoperative rehabilitation
Because these are small tears that risk leading to a frozen shoulder, immediate passive mobilization is recommended. Moreover, because they are unretracted, wearing a simple sling for a maximum of one month should suffice. Once the range of joint movement is re-acquired and allowing for a 6-week healing period, then gradual return to activities favourable to the rotator cuff, and which protect the subacromial space, is recommended; for instance walking with a stick, rowing, elliptical machine, paddle and breaststroke.[43]
References
- ↑ 1.0 1.1 Ellman H. Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop Relat Res 1990:64-74.
- ↑ 2.0 2.1 2.2 Codman E. Lesions of the supraspinatus tendon and other lesions in or about the subacromial bursa. In: Co. TT, ed. The Shoulder. Boston, MA1934:65-7.
- ↑ Clavert P, Le Coniat Y, Kempf JF, Walch G. Intratendinous rupture of the supraspinatus: anatomical and functional results of 24 operative cases. European journal of orthopaedic surgery & traumatology : orthopedie traumatologie 2016;26:133-8.
- ↑ Walz DM, Miller TT, Chen S, Hofman J. MR imaging of delamination tears of the rotator cuff tendons. Skeletal radiology 2007;36:411-6.
- ↑ Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg Am 1995;77:10-5.
- ↑ Smith C, Corner T, Morgan D, Drew S. Partial thickness rotator cuff tears: what do we know? Shoulder Elbow 2010;2:77-82.
- ↑ Fukuda H. The management of partial-thickness tears of the rotator cuff. J Bone Joint Surg Br 2003;85:3-11.
- ↑ Kim YS, Kim SE, Bae SH, Lee HJ, Jee WH, Park CK. Tear progression of symptomatic full-thickness and partial-thickness rotator cuff tears as measured by repeated MRI. Knee Surg Sports Traumatol Arthrosc 2017;25:2073-80.
- ↑ Kong BY, Cho M, Lee HR, Choi YE, Kim SH. Structural Evolution of Nonoperatively Treated High-Grade Partial-Thickness Tears of the Supraspinatus Tendon. Am J Sports Med. 2018;46:79-86.
- ↑ Kuhn JE, Dunn WR, Ma B, Wright RW, Jones G, Spencer EE, Wolf B, Safran M, Spindler KP, McCarty E, Kelly B, Holloway B Interobserver agreement in the classification of rotator cuff tears. Am J Sports Med 2007;35:437-41.
- ↑ Mochizuki T, Sugaya H, Uomizu M, Maeda K, Matsuki K, Sekiya I, Muneta T, Akita K. Humeral insertion of the supraspinatus and infraspinatus. New anatomical findings regarding the footprint of the rotator cuff. J Bone Joint Surg Am 2008;90:962-9.
- ↑ Lee SB, Nakajima T, Luo ZP, Zobitz ME, Chang YW, An KN. The bursal and articular sides of the supraspinatus tendon have a different compressive stiffness. Clinical biomechanics 2000;15:241-7.
- ↑ Pouliart N, Somers K, Eid S, Gagey O. Variations in the superior capsuloligamentous complex and description of a new ligament. J Shoulder Elbow Surg 2007;16:821-36.
- ↑ Gerber C, Zubler V, Hodler J, Catanzaro S, Jost B, Fucentese SF. Dynamic imaging and function of partial supraspinatus tendon tears. Arthroscopy 2011;27:1180-6.
- ↑ Pinkowsky GJ, ElAttrache NS, Peterson AB, Akeda M, McGarry MH, Lee TQ. Partial-thickness tears involving the rotator cable lead to abnormal glenohumeral kinematics. J Shoulder Elbow Surg 2017;26:1152-8.
- ↑ Rudzki JR, Adler RS, Warren RF, Kadrmas WR, Verma N, Pearle AD, Lyman S, Fealy S. Contrast-enhanced ultrasound characterization of the vascularity of the rotator cuff tendon: age- and activity-related changes in the intact asymptomatic rotator cuff. J Shoulder Elbow Surg 2008;17:96S-100S.
- ↑ Poldoja E, Rahu M, Kask K, Weyers I, Kolts I. Blood supply of the subacromial bursa and rotator cuff tendons on the bursal side. Knee Surg Sports Traumatol Arthrosc 2017;25:2041-6.
- ↑ Zumstein M, Lädermann A, Raniga S, Schaer M. The Biology of Rotator Cuff Healing. Orthop Traumatol Surg Res. 2017;103(1S):S1-S10.
- ↑ Oh JH, Oh CH, Kim SH, Kim JH, Yoon JP, Jung JH. Clinical features of partial anterior bursal-sided supraspinatus tendon (PABST) lesions. J Shoulder Elbow Surg 2012;21:295-303.
- ↑ Walch G, Liotard JP, Boileau P, Noël E. [Postero-superior glenoid impingement. Another shoulder impingement]. Rev Chir Orthop Reparatrice Appar Mot. 1991;77:571-4.
- ↑ 21.0 21.1 Lädermann A, Chagué S, Kolo FC, Charbonnier C. Kinematics of the shoulder joint in tennis players. J Sci Med Sport. 2016;19:56-63.
- ↑ Moor BK, Wieser K, Slankamenac K, Gerber C, Bouaicha S. Relationship of individual scapular anatomy and degenerative rotator cuff tears. J Shoulder Elbow Surg 2014;23:536-41.
- ↑ Kim SH, Ha KI. Arthroscopic treatment of symptomatic shoulders with minimally displaced greater tuberosity fracture. Arthroscopy 2000;16:695-700.
- ↑ Collin P, Treseder T, Denard PJ, Neyton L, Walch G, Lädermann A. What is the Best Clinical Test for Assessment of the Teres Minor in Massive Rotator Cuff Tears? Clin Orthop Relat Res 2015;473:2959-66.
- ↑ Kelly SM, Brittle N, Allen GM. The value of physical tests for subacromial impingement syndrome: a study of diagnostic accuracy. Clinical rehabilitation 2010;24:149-58.
- ↑ 26.0 26.1 Brockmeyer M, Schmitt C, Haupert A, Kohn D, Lorbach O. Limited diagnostic accuracy of magnetic resonance imaging and clinical tests for detecting partial-thickness tears of the rotator cuff. Arch Orthop Trauma Surg. 2017;137:1719-1724.
- ↑ Kurz AZ, Kelly MJ, Hackett L, Murrell GA. Effect of surgeon-sonographer interaction on ultrasound diagnosis of rotator cuff tears: a five-year cohort study in 775 shoulders. J Shoulder Elbow Surg 2016;25:1385-94.
- ↑ Plomb-Holmes C, Clavert P, Kolo F, Tay E, Lädermann A, French Society of A. An orthopaedic surgeon's guide to ultrasound imaging of the healthy, pathological and postoperative shoulder. Orthop Traumatol Surg Res. 2018;104(8S):S219-S232
- ↑ 29.0 29.1 29.2 29.3 Lädermann A, Collin P. Rupture partielle de la coiffe des rotateurs postéro-supérieur. Revue du Rhumatisme Monographies 2018;85:88-94.
- ↑ de Jesus JO, Parker L, Frangos AJ, Nazarian LN. Accuracy of MRI, MR arthrography, and ultrasound in the diagnosis of rotator cuff tears: a meta-analysis. AJR American journal of roentgenology 2009;192:1701-7.
- ↑ Schaeffeler C, Mueller D, Kirchhoff C, Wolf P, Rummeny EJ, Woertler K. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. European radiology 2011;21:1477-84.
- ↑ Kempf JF, Mole D, Gleyze P, Bonnomet F, Rio B, Levigne C, Walch G. [Results of endoscopic treatment of tendinopathies of the rotator cuff (excluding total ruptures). 1: Non-calcifying tendinopathies]. Rev Chir Orthop Reparatrice Appar Mot 1993;79:519-31.
- ↑ 33.0 33.1 Kamath G, Galatz LM, Keener JD, Teefey S, Middleton W, Yamaguchi K. Tendon integrity and functional outcome after arthroscopic repair of high-grade partial-thickness supraspinatus tears. J Bone Joint Surg Am 2009;91:1055-62.
- ↑ Schwitzguebel AJ, Kolo FC, Tirefort J, Kourhani A, Nowak A, Gremeaux V, Saffarini M, Lädermann A. Efficacy of Platelet-Rich Plasma for the Treatment of Interstitial Supraspinatus Tears: A Double-Blinded, Randomized Controlled Trial. Am J Sports Med. 2019;47:1885-1892.
- ↑ Katthagen JC, Bucci G, Moatshe G, Tahal DS, Millett PJ. Improved outcomes with arthroscopic repair of partial-thickness rotator cuff tears: a systematic review. Knee Surg Sports Traumatol Arthrosc 2018;26:113-124.
- ↑ Xiao J, Cui G. Clinical and structural results of arthroscopic repair of bursal-side partial-thickness rotator cuff tears. J Shoulder Elbow Surg 2015;24:e41-6.
- ↑ Park SE, Panchal K, Jeong JJ, Kim YY, Kim JH, Lee JY, Ji JH. Intratendinous rotator cuff tears: prevalence and clinical and radiological outcomes of arthroscopically confirmed intratendinous tears at midterm follow-up. Am J Sports Med 2015;43:415-22.
- ↑ 38.0 38.1 Shin SJ. A comparison of 2 repair techniques for partial-thickness articular-sided rotator cuff tears. Arthroscopy 2012;28:25-33.
- ↑ Gonzalez-Lomas G, Kippe MA, Brown GD, Gardner TR, Ding A, Levine WN, Ahmad CS. In situ transtendon repair outperforms tear completion and repair for partial articular-sided supraspinatus tendon tears. J Shoulder Elbow Surg 2008;17:722-8.
- ↑ Peters KS, Lam PH, Murrell GA. Repair of partial-thickness rotator cuff tears: a biomechanical analysis of footprint contact pressure and strength in an ovine model. Arthroscopy 2010;26:877-84.
- ↑ Gerber C, Catanzaro S, Betz M, Ernstbrunner L. Arthroscopic Correction of the Critical Shoulder Angle Through Lateral Acromioplasty: A Safe Adjunct to Rotator Cuff Repair. Arthroscopy 2018;34:771-780.
- ↑ Levigne C, Garret J, Grosclaude S, Borel F, Walch G. Surgical technique arthroscopic posterior glenoidplasty for posterosuperior glenoid impingement in throwing athletes. Clin Orthop Relat Res 2012;470:1571-8.
- ↑ Charbonnier C, Lädermann A, Kevelham B, Chague S, Hoffmeyer P, Holzer N. Shoulder strengthening exercises adapted to specific shoulder pathologies can be selected using new simulation techniques: a pilot study. Int J Comput Assist Radiol Surg. 2018;13:321-330.