Difference between revisions of "Posterosuperior Rotator Cuff Tears and Associated Pathologies"
| Line 259: | Line 259: | ||
'''''<small>Table: Different Classification of Massive Rotator Cuff Tears</small>''''' | '''''<small>Table: Different Classification of Massive Rotator Cuff Tears</small>''''' | ||
| − | [[File:1562478200580-lg.jpg|thumb| | + | [[File:1562478200580-lg.jpg|thumb|600x600px|alt=|center]] |
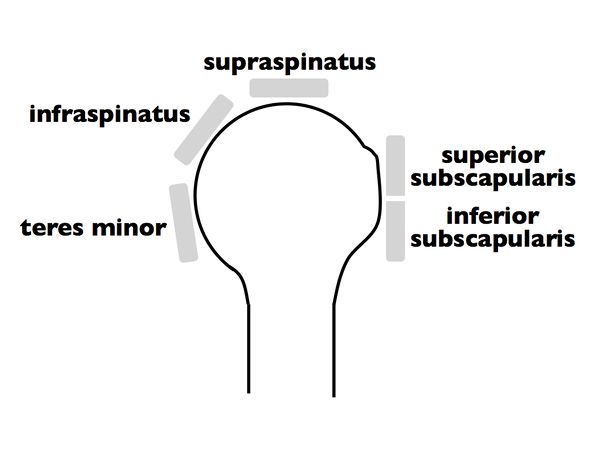
<br>Once a massive rotator cuff tear is identified, it can be further classified according to Collin et al.2 In this subclassification, the rotator cuff is divided into five components: supraspinatus, superior subscapularis, inferior subscapularis, infraspinatus, and teres minor (Figure). | <br>Once a massive rotator cuff tear is identified, it can be further classified according to Collin et al.2 In this subclassification, the rotator cuff is divided into five components: supraspinatus, superior subscapularis, inferior subscapularis, infraspinatus, and teres minor (Figure). | ||
[[File:1562478202097-lg.jpg|center|thumb|600x600px|Figure. 21 The rotator cuff is divided into 5 components: supraspinatus, superior subscapularis, inferior subscapularis, infraspinatus, and teres minor.]] | [[File:1562478202097-lg.jpg|center|thumb|600x600px|Figure. 21 The rotator cuff is divided into 5 components: supraspinatus, superior subscapularis, inferior subscapularis, infraspinatus, and teres minor.]] | ||
Revision as of 15:11, 3 January 2020
Contents
- 1 Bullet Points
- 2 Key Words
- 3 Biomechanics of the Posterosuperior Rotator Cuff
- 4 Clinical examination
- 5 Imaging
- 6 Classification
- 6.1 Type A: Bony Involvement
- 6.2 A.3 Tuberosity Insufficiency
- 6.3 Surgical Technique
- 6.4 Type B: Full Thickness Tendon Lesion
- 6.4.1 B1: Lateral tendinous disruption
- 6.4.1.1 Size of Tendon Lesion
- 6.4.1.2 Tendon Retraction
- 6.4.1.3 Tear Pattern
- 6.4.1.4 Releases for the Rotator Cuff
- 6.4.1.5 Double-Row Versus Single-Row Cuff Repair
- 6.4.1.6 Margin Convergence
- 6.4.1.7 Load Sharing Rip Stop Construct
- 6.4.1.8 Massive Posterosuperior Rotator Cuff Tears
- 6.4.1.9 Definition and Classification of Massive Rotator Cuff Tears
- 6.4.1.10 Suprascapular Nerve Neuropathy and Massive Rotator Cuff Tear
- 6.4.2 Treatment Options for Massive Rotator Cuffs
- 6.4.3 B2: Medial Tendinous Disruption
- 6.4.1 B1: Lateral tendinous disruption
- 6.5 B3: Tendon to Tendon Adhesion: “Fosbury Flop Tear”
- 6.6 Type C: Musculotendinous Junction Lesion
- 6.7 Type D: Muscle Insufficiency
- 7 Irreparable Rotator Cuff Tears
- 8 References
Bullet Points
- The rotator cable explains why patients with most rotator cuff tears can maintain active forward flexion, and also why even after only a partial rotator cuff repair, good functional results can be achieved.
- The most important negative prognostic factor is high-grade fatty infiltration of the rotator cuff muscle bellies (grade 3 or 4 fatty infiltration).
- The tangent sign is an indicator of advanced fatty infiltration and is a predictor of whether a rotator cuff tear will be reparable.
- Full thickness disruption of the lateral tendon stump (B1) is the most frequent type of rotator cuff lesion, comprising approximately 90% of all surgically treated lesions.
- Musculotendinous junction lesions (C-type) or rare and characterized by an edema of the muscle belly. They are associated to calcific deposit (infraspinatus) or trauma (supraspinatus). Unrepaired, grade III lesions lead rapidly to grade 4 fatty infiltration of the muscle.
- Tendon retraction is classified according to Patte. Overreduction and lateral transposition of the tendon over the greater tuberosity may be unphysiological.
- Massive rotator cuff has different definitions in the literature, each having potential benefits or drawbacks.
- Massive rotator cuff tears comprise approximately 20% of all cuff tears and 80% of recurrent tears.
- The classification of Collin not only subclassifies massive tears but has also been linked to function, particularly the maintenance of active elevation.
- Non-surgical treatment is effective in patient with massive rotator cuff if the tear involves less than three tendons and do not involves the subscapularis (D-type).
- Biomechanical testing has consistently demonstrated the superiority of double-row constructs over single-row. However, there is no obvious difference clinically.
- There is actually no support for routine suprascapular nerve release when massive rotator cuff repair is performed.
- Functional outcome improved after revision rotator cuff repair and 70% or more of patients were satisfied or very satisfied. However, the prevalence of persistent defect (retear or non-healing) is 28% at six months and 40% at two years.
- Rotator cuff are irreparable when associated to true pseudoparalysis with the presence of lag signs (external rotation lag, drop, dropping, hornblower signs), femoralization of the humerus or acetabulization of the acromion, grade 3 or 4 fatty infiltration and tangent sign.
- The current literature does not support the initial use of complex and expensive techniques in the management of posterosuperior irreparable rotator cuff tears.
Key Words
Shoulder arthroscopy; Rotator cuff lesion; Partial repair; Tear pattern; Classification; Massive; Reparable and non-repairable; Irreparable; Imaging; Recurrent; Failed; Revision surgery; Open and arthroscopic approach; Conservative or non-operative treatment; Physiotherapy; Functional outcomes; Prognostic factors; Latissimus dorsi transfer; Subacromial spacer interposition; Balloon; Biceps tenotomy; Superior capsular reconstruction; Reverse arthroplasty; Magnetic resonance imaging (MRI) arthrography (MRA); Fosbury flop tear; New tear pattern; FUSSI; SAM.
Biomechanics of the Posterosuperior Rotator Cuff
A primary function of the rotator cuff is to work synergistically with the deltoid to maintain a balanced force couple about the glenohumeral joint. A force couple is a pair of forces that act on an object and tend to cause it to rotate. For any object to be in equilibrium, the forces must create moments about a center of rotation that are equal in magnitude and opposite in direction. Coronal and transverse plane force couples exist between the subscapularis anteriorly and infraspinatus and teres minor posteriorly. The rotator cuff force across the glenoid provides concavity compression, which creates a stable fulcrum and allows the periscapular muscles to move the humerus around the glenoid.
The rotator cable is a thickening of the rotator cuff that has been likened to a suspension bridge in which force is distributed through cables that are supported by pillars (the anterior and posterior attachments). The anterior rotator cable attachment bifurcates to attach to bone just anterior and posterior to the proximal aspect of the bicipital groove. The posterior attachment comprises the inferior 50% of the infraspinatus. With small central tears the cable attachments often stay intact and forces are transmitted along the rotator cable. The rotator cable also explains why patients with most rotator cuff tears can maintain active forward flexion, and also why even after only a partial rotator cuff repair, good functional results can be achieved.[1]
However, in the setting of massive rotator cuff with rotator cable disruption and non-compensation by other humeral head stabilizers (i.e pectoralis major and latissimus dorsi), the moments created by the opposing muscular forces are insufficient to maintain equilibrium in the coronal plane, resulting in altered kinematics, instability, and ultimately in pseudoparalysis. Interestingly, only few patients with an irreparable rotator cuff tears developed pseudoparalysis and arthritis.This finding has at least two potential explanations. First, the subscapularis that may not be involved in these tears is the key factor of active forward flexion.[2]
Second, the rotator cable, has still an intact anterior attachment which is important for elevation. This may explain why patients can maintain active mobility, and also why even after only a partial rotator cuff repair, good functional results can be achieved.[3]
Consequently, all the conditions for an imbalance in the force couples are not always met and subsequently loss of function is only occasionally seen.
Clinical examination
Supraspinatus
Superior rotator cuff insufficiency, present in complete tears, is usually associated with a positive Jobe manoeuver and decreased strength in the external resistance of the elbow at the side.[4]
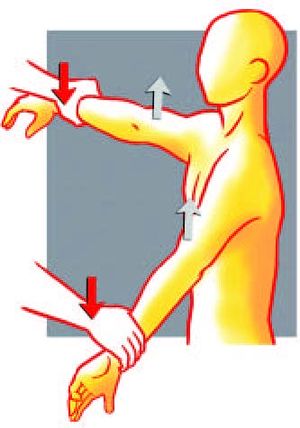
Figure. 1 Jobe manoeuver: the examiner push both arms down at the level of the wrists. Reproduce from Liotard J, Walch G. Test de Jobe. Recherche d'une atteinte du tendon supraépineux. In: Rodineau J, ed. 33 tests incontournables en traumatologie du sport. Paris: Éd. scientifiques; 2009, with permission.
Testing of abduction strength in the champagne toast position, i.e., 30° of abduction, mild external rotation, and 30° of flexion, better isolates the activity of the supraspinatus from the deltoid than Jobe's “empty can” position (Figure).[5]
Infraspinatus and Teres Minor
Strength in External Rotation Elbow at the Side
Strength in external rotation elbow at the side of the supraspinatus, infraspinatus and teres minor represents approximately 10%, 70% and 20% of total external rotation strength, respectively.[6]
However, the function of the teres minor may become more important in the setting of a chronic infraspinatus tear, as its hypertrophy is commonly observed in these cases and probably compensates for external rotation weakness.
External Rotation Lag Sign
The external rotation lag sign (Figure and Video), described by Hertel, was designed to test the integrity of infraspinatus and supraspinatus tendons.[7]
The extent of internal rotation is recorded to the nearest 10 degrees degrees (10, 20, 30 and 40 degrees or above). An external rotation lag sign > 40 degrees seems to be the most reliable test for the teres minor.[8]
Drop Sign
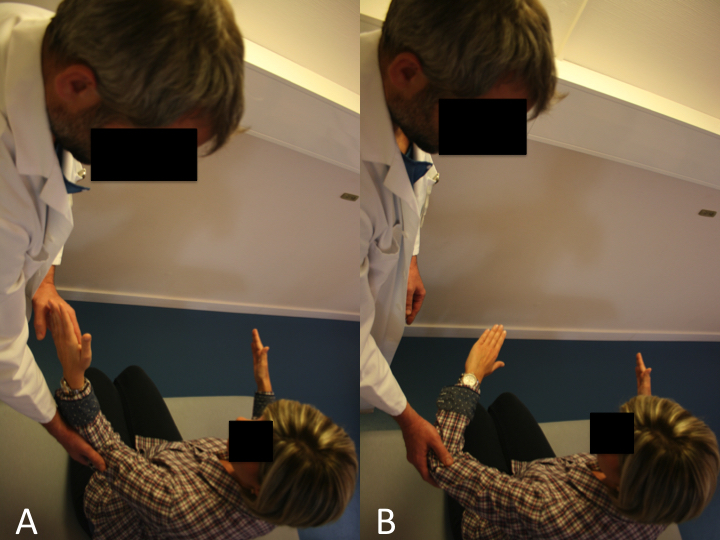
The drop sign (Figure and Video), also described by Hertel, is designed to assess the function of the infraspinatus.
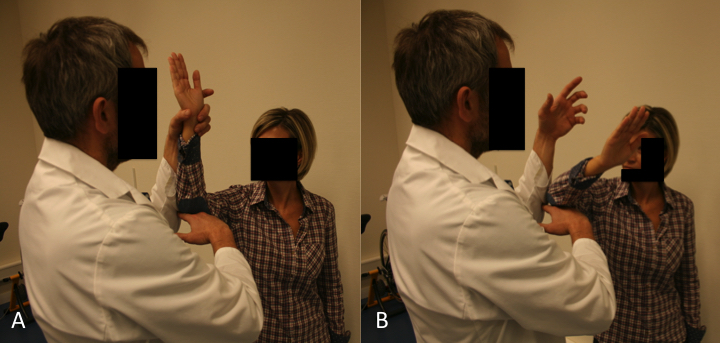
A) The drop sign is a lag sign beginning from 90 degrees of abduction in the scapular plane, with elbow flexion of 90 degrees, and external rotation of the shoulder to 90 degrees. From this position, the patient is asked to maintain the position against gravity (MRC Grade 3).
B) Failure to resist gravity and internal rotation of the arm is considered a positive drop sign. Reproduce from Collin et al., with permission.
Hornblower sign
The patient is asked to bring both hands to his mouth, but is unable to do so without abducting the affected arm (Video).
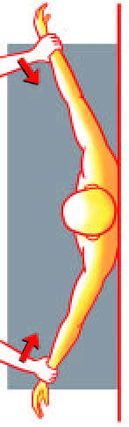
Patte Test
The Patte test (Figure and Video) is the only test that allowed to analyze the muscular strength of the teres minor in case of deficient infraspinatus.[9]

A) The Patte test is performed by passively taking from a starting point of 90 degrees of abduction in the scapular plane, an elbow flexion of 90 degrees without external rotation.
B) The patient is asked to perform external rotation of the shoulder from this position against resistance. A positive Patte test is defined as external rotation power less than MRC Grade 4. Reproduce from Collin et al., with permission.
Walch et al. reported a 100% sensitivity and 93% specificity with the Patte test and teres minor fatty atrophy Grade 3 or greater.[10]
Dropping Sign
The dropping sign of Neer had a 100% sensitivity and 66% specificity for teres minor involvement.[11][12]
Imaging
X-rays
The analysis should always begin with plain radiographic views to determine the morphology and status of the glenohumeral joint to exclude glenohumeral arthritis:
Anteroposterior True anteroposterior X-ray with the arm in neutral rotation, and the patient relaxed is obtained to evaluate the shape of the acromion and greater tuberosity, the critical shoulder angle, and the acromiohumeral distance. A decreased acromiohumeral distance < 7 mm in a standard antero-posterior radiograph indicates superior migration of the humeral head which increases the probability of finding an irreparable cuff tear. Such distance is correlated to 1) tears of the infraspinatus that mainly acts in lowering the humeral head, and 2) varying degrees of fatty infiltration.[13][14]
Nevertheless, such criteria should be interpreted with parsimony. First, it is difficult in clinical practice to obtain standardized X-rays making measurement aleatory. Second, this distance has not been associated with an inability to obtain an intra-operative complete repair of the supraspinatus (18.2% irreparable, OR = 0.55, P = 0.610).[15]
At the end of the spectrum, acetabularization of the acromion and femoralization of the humeral head are pre-operative adapting factors reflecting significant chronic static superior instability and are a contraindication for repair.
Lateral Y-view (Lamy)
Lateral Y-view (Lamy) is used to analyze the presence of a spur, the shape of the acromion on this view is less accurate to detect full-thickness rotator cuff tear.[16]
Axillary lateral
An axillary lateral view can exclude static anterior subluxation or os acromialis.
If pathology of the acromioclavicular joint is suspected, a Zanca view is additionally acquired.[17]
Ultrasound (US)
Following X-ray evaluation, advanced imaging modalities are obtained to confirm and plan treatment. Ultrasonography is an excellent cost-effective screening tool in the office but does not allow evaluation of intra-articular pathology or easy evaluation of muscle quality.
Rotator Cuff Interval
The space through which the long head of the biceps passes as it leaves the glenohumeral joint is called the rotator cuff interval. The patient position is the same as for evaluation of the long head of the biceps, with the probe being placed slightly superiorly to the bicipital groove and in the axial plane (Figure). The long head of the biceps is thus visualized with the subscapularis medially and the supraspinatus laterally, while the coracohumeral and superior glenohumeral ligaments surround it.[18]
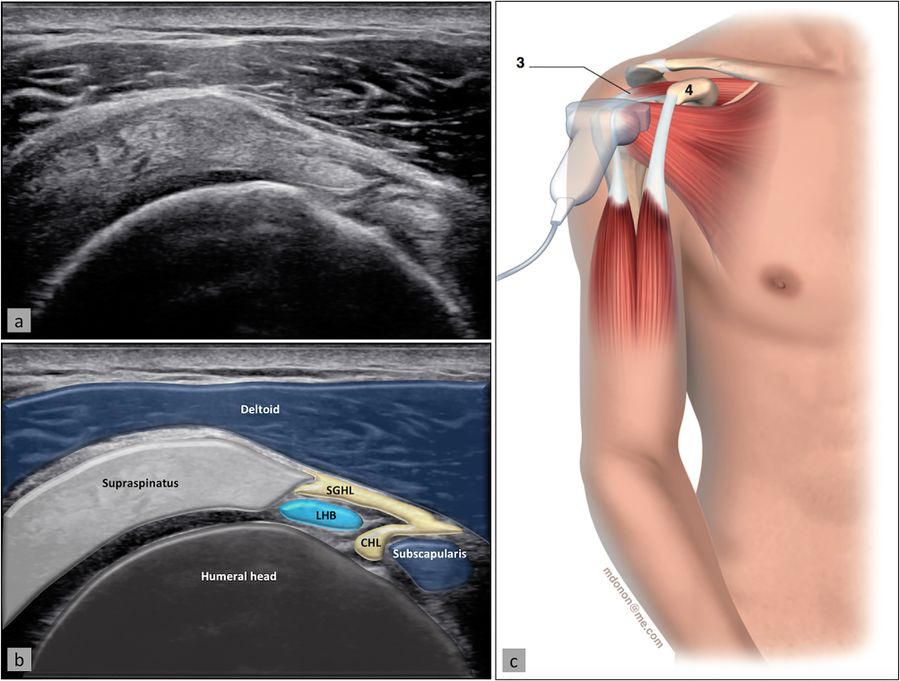
Supraspinatus Tendon and Subacromial-Subdeltoid Bursa
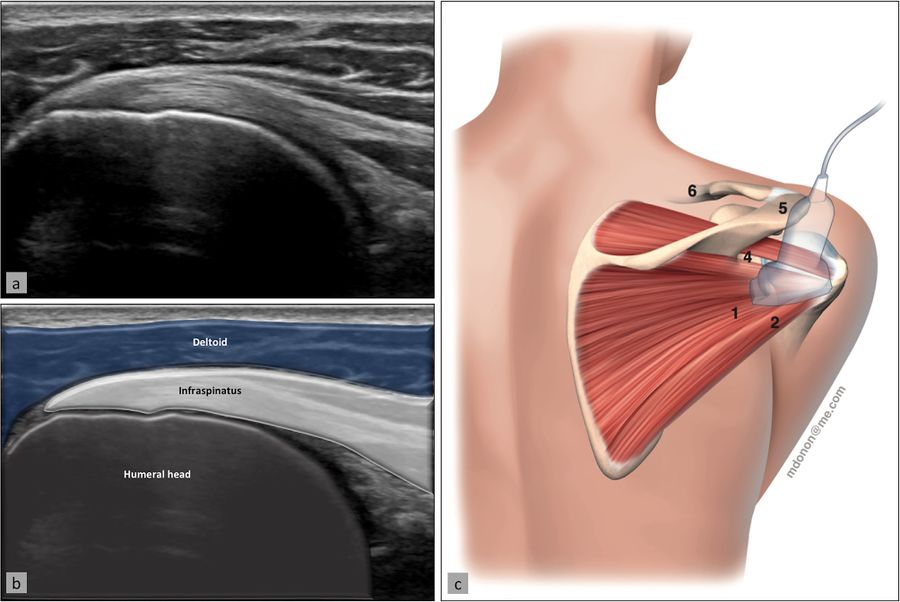
The supraspinatus tendon is best visualized with the shoulder in abduction and internal rotation, by asking the patient to place the palm of their hand on their back pocket, elbow pointed backwards (Figure). In patients presenting with reduced range of motion (adhesive capsulitis for example), maximal internal rotation with the arm hanging by the side of the thorax can be sufficient. The long axis of the tendon is most useful for analyzing integrity of the tendon on the footprint (measuring approx. 2 cm medially to laterally), and is visualized by holding the probe in a tilted position (therefore not a true coronal plane but at an approx. 45 degree angle, following the line of the humerus (Figure)).
This position also allows visualization of two other structures: the subacromial-subdeltoid bursa (and the presence of excessive liquid, see below) and the humeral head along with its articular cartilage (and possible surface defects). In the axial plane (again not truly axial but at 90 degrees to the previous plane), the leading edge of the supraspinatus can be identified laterally to the biceps tendon. Moving the probe laterally will reveal the mid-portion of the tendon, with the anterior part of the infraspinatus eventually coming into view as an anisotropic and dark image (as the fibers run in a different plane).
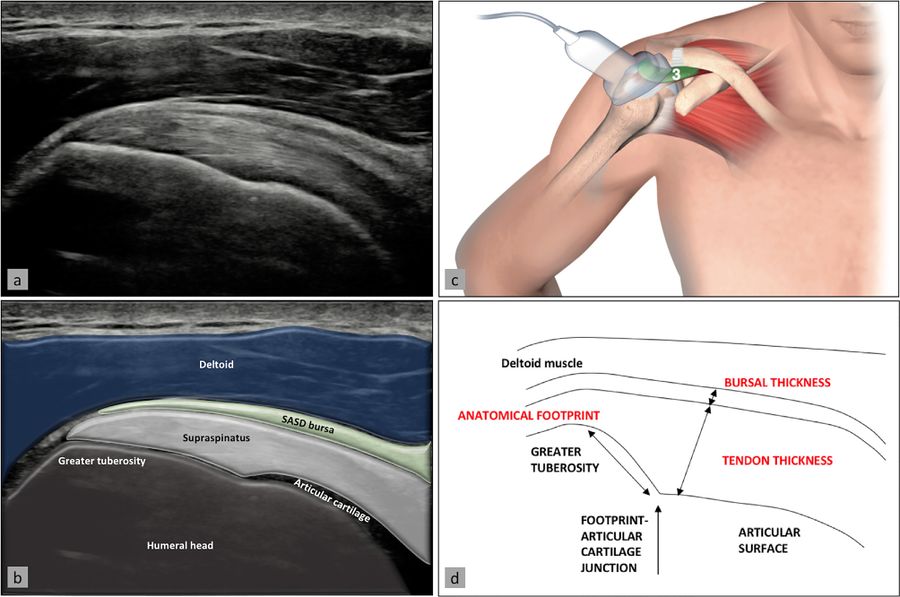
Ultrasound image (a) with superimposed anatomy (b), patient/probe position (c), and landmarks for measurement of these two structures (d). Reproduced from Plomb-Holmes et al., with permission
Infraspinatus and teres minor tendon, glenohumeral joint, spinoglenoid notch
The infraspinatus tendon, which inserts posteriorly to the supraspinatus tendon, is best examined in its long axis by elongating it (the patient placing his or her hand on the opposite shoulder) and placing the probe on the posterior part of the patient’s shoulder (Figure). The insertion of the tendon on the humeral head can be analyzed, as well as the musculotendinous junction by sliding the probe medially. At this point, the glenohumeral joint line and posterior labrum can be visualized in thin patients, and even more medially, the spinoglenoid notch containing the suprascapular neurovascular bundle (and the possible presence of a ganglion cyst arising from the posterior labrum which can compress the bundle) (Figure). The teres minor tendon can be difficult to separate from the infraspinatus tendon; it is located inferiorly and has a similar aspect, but can be distinguished by the fact that deeper to it lies bone whereas the infraspinatus lies on articular cartilage, and its insertion is primarily muscular (vs. tendinous).
Magnetic Resonance Imaging (MRI) and Computer Tomography (CT)
Magnetic resonance imaging accurately estimates tear pattern, muscle fatty infiltration and atrophy, tendon length and retraction, and is thus obtained to plan repair or reconstructive surgeries. The muscle bellies of the rotator cuff are assessed, if available, on T1-weighted axial, coronal, sagittal views with cuts sufficiently medial on the scapula to allow proper assessment regardless of retraction. Finally, computer tomography (CT) scans are used if magnetic resonance imaging is contraindicated or if joint replacement is planned, particularly in the setting of glenoid deformity. Additionally, computer tomography (CT) scan can be conducted with intra-articular contrast to assess the cuff. It should be noted that the magnetic resonance imaging and computer tomography are not reliable to analyze the acromiohumeral distance as they are performed in lying position.
Fatty Infiltration
The most important negative prognostic factor is high-grade fatty infiltration of the rotator cuff muscle bellies (grade 3 or 4 fatty infiltration) (Figure).
Fatty infiltration is irreversible even with repair and leads to reduced function of the rotator cuff musculature.[19]
If pathology of the acromioclavicular joint is suspected, a Zanca view is additionally acquired.[20][21][22]
Atrophy
The presence or absence of supraspinatus atrophy is determined using the tangent sign of Zanetti et al. (Figure).[23]
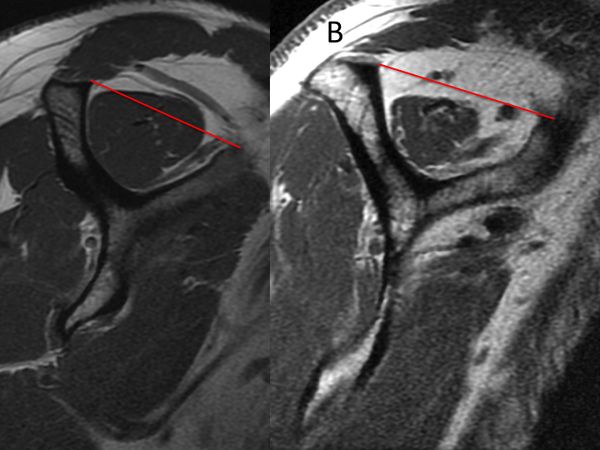
This sign is an indicator of advanced fatty infiltration and has been reported to be a predictor of whether a rotator cuff tear will be reparable.[24][25]
An inability to obtain a complete repair of the supraspinatus is associated with a positive tangent sign (30% irreparable) versus a negative tangent sign (6.3% irreparable, OR = 6,3, P =0.0102).[26]
Supraspinatus atrophy can also be determine according to Thomazeau classification.[27]
Agreement for this classification is however fair (intra-observer kappa = 0,51 and inter-observer kappa = 0.30) and its use cannot be recommended as a criteria of reparability.[28]
Classification
Reproduced from Lädermann A, Burkhart SS, Hoffmeyer P, et al. Classification of full-thickness rotator cuff lesions: a review. EFORT Open Rev 2016;1:420-30, with permission
The rotator cuff lesions are categorized into four major groups based on involvement of the bone (Type A), tendon (Type B), musculotendinous junction (Type C) or muscle insufficiency (Type D).
Type A: Bony Involvement
While the majority of rotator cuff lesions involve the tendinous insertion, bony involvement is an important consideration. Bony involvement includes acute fractures, malunion/nonunion, and chronic bony insufficiency.
A1. Acute Bony Involvement (Fractures and Avulsions)
Isolated greater tuberosity fractures are considered uncommon, representing less than 5% of all operatively treated proximal humeral fractures.[29]
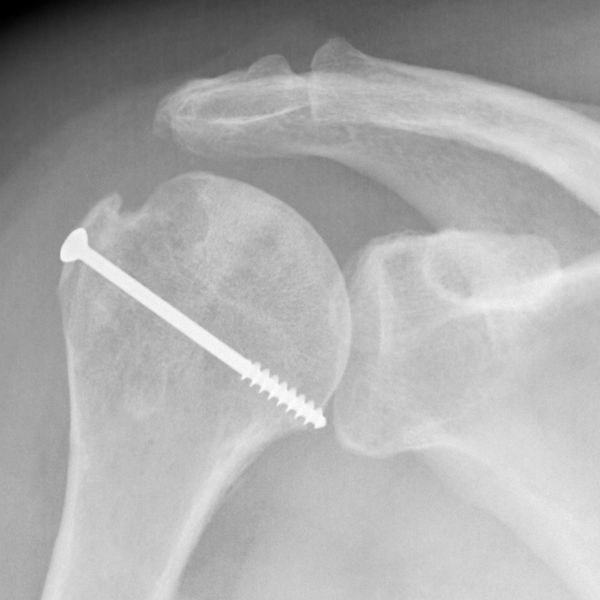
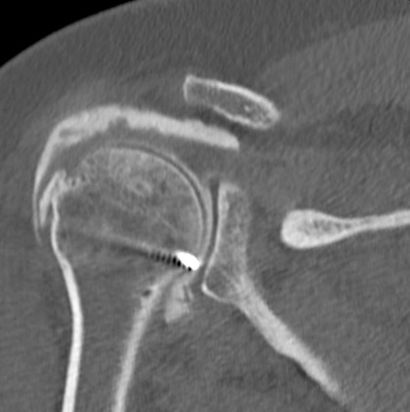
Isolated lesser tuberosity fractures are generally considered rare. Type A lesion of the greater or lesser tuberosity represent approximately 3.2% and 1.1% respectively of surgically treated rotator cuff lesions (Table). Tuberosity fractures are included in the accepted classification for proximal humeral fractures by Neer, in itself a modification of Codman’s original description. Because the greater and lesser tuberosity are the insertion site of the rotator cuff, even small tuberosity fractures or avulsions can represent substantial disruption of the rotator cuff and lead to functional impairment if displaced and left untreated. Historically, Neer proposed 10 mm of displacement as a threshold for operative intervention.[30]
However, more recent investigation has recommended that a threshold of 5 mm31 should be used.[31]
Displacement of greater than 5 mm can lead to bony impingement with loss of range of motion as well as loss of strength from compromise in the normal length–tension relationship of the rotator cuff. A traumatic mechanism is typical such as violent muscular contraction, impaction of the greater tuberosity beneath the acromion, or shearing against the anterior glenoid rim during a glenohumeral dislocation event. Thorough patient evaluation is required to make an appropriate treatment recommendation. Conservative therapy is limited to non- or minimally-displaced fractures. The ongoing development of arthroscopic techniques has led to multiple reports about arthroscopically assisted or total arthroscopic techniques in the treatment of these injuries.[32]
A.2 Tuberosity Malunion/Nonunion
Tuberosity malunion or nonunion can be a sequela of either conservative treatment or surgical treatment of acute injuries. As noted previously, displacement effectively shortens the muscle-tendon unit such that the rotator cuff cannot function properly (Figures).
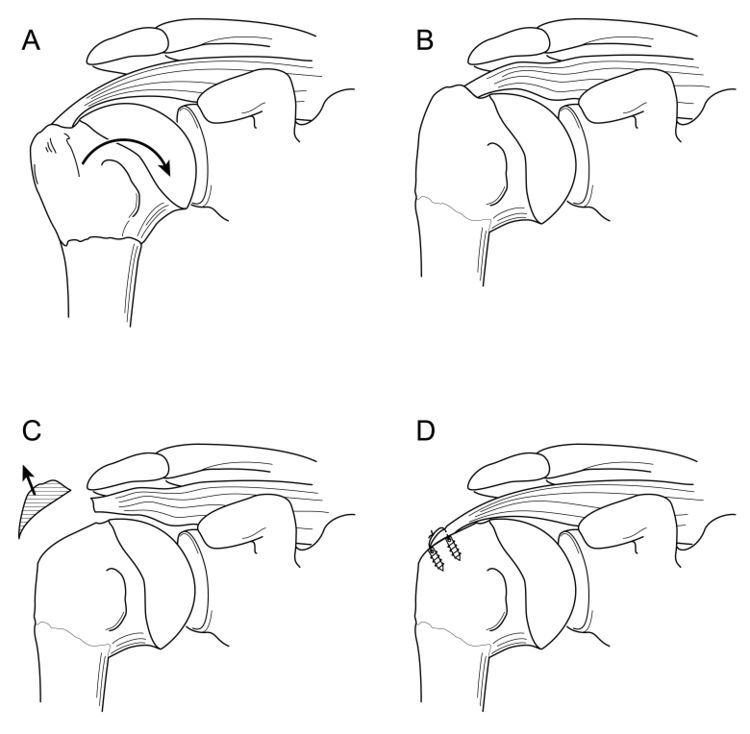
Various open techniques have been described for the management of the malunion of proximal humeral fractures, including prosthetic reconstruction, open corrective osteotomy, or arthroscopic capsular release followed by takedown of the rotator cuff from the malunited proximal humerus, tuberoplasty, and then rotator cuff advancement. Although the latter technique is technically demanding, it allows preservation of the native humeral head, which is associated with a low complication rate, and avoids concerns about long-term prosthetic survival in young patients (Video).[33][34]
Operative Technique
The patient was placed in a beach chair or in a lateral decubitus position. A diagnostic arthroscopy is performed with an arthroscopic pump maintaining pressure at 50 mm Hg. The joint surfaces were inspected to rule out any incongruities. The articular side of the rotator cuff is carefully assessed with a probe searching for tears. Any capsular retraction is addressed at this point. A release of the rotator interval and superior glenohumeral ligament is performed with an electrocautery introduced from an anterior portal. This was followed by a release of the posterior, inferior, and anterior capsule 5 mm away from the labrum with an electrocautery introduced from the posterior portal while the surgeon is viewing from an anterosuperolateral portal. If still present, the intra articular part of the long head of the biceps tendon undergo either tenotomy or tenodesis. After treatment of any intra-articular pathology, such as loose body removal, attention is turned to the subacromial space.
The lateral and posterolateral gutters are cleared. ny previously placed metal hardware are removed. While the surgeon is viewing from a posterior glenohumeral portal, the tuberoplasty is initiated. The arthroscope is then moved to the subacromial space, and the rotator cuff, if necessary, is sharply elevated from its malunited footprint by use of an electrocautery. Elevation of the rotator cuff consisted of the supraspinatus and anterior half of the infraspinatus, which is the part that overlies the proximally migrated tuberosity (Video).
After elevation of the rotator cuff attachments, a burr is used to perform a tuberoplasty. The cuff is assessed for mobility and integrity and is then retensioned by advancing the cuff laterally on the greater tuberosity and performing a rotator cuff repair. The rotator cuff is advanced and repaired. A modified acromioplasty with a lateral bevel is routinely performed if not done previously.
A.3 Tuberosity Insufficiency
Tuberosity insufficiency can range from contained cystic bony defects within the tuberosity to the absence of the entire tuberosity. Cystic bony defects are often encountered during primary or revision rotator cuff repair. Such defects may be idiopathic, related to a patient's rotator cuff disease, or secondary to osteolysis from breakdown of bioreabsorbable anchors. These osseous defects reduce biological healing capacity and may decrease repair fixation strength. Bone grafting techniques are needed to address these defects.[35]
In such a situation, a simple tendon rotator repair is usually unsuccessful, as a large bony defect significantly lowers the prognosis for primary repair.[36]
One explanation might be that deltoid tension and therefore function is potentiated by the greater tuberosity, also called “deltoid wrapping”.[37]
Therefore, reconstruction of this combined bony and tendon defect may require both tendinous and bony reconstruction. In older patients, such insufficiency is most reliably addressed with reverse shoulder arthroplasty. However, reverse shoulder arthroplasty is not ideal for young patients as multiple studies have demonstrated increased complications in this patient population.[38][39]
Recently, a fresh frozen bony-tendinous allograft of the calcaneus and Achilles tendon has been proposed to address this difficult problem (Video). Long term results and larger series need to confirm this technique.[40]
Surgical Technique
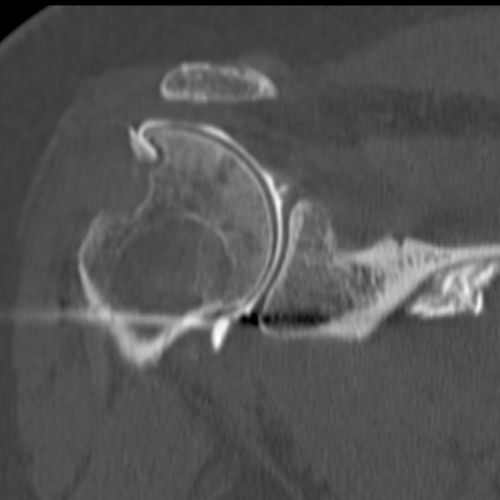
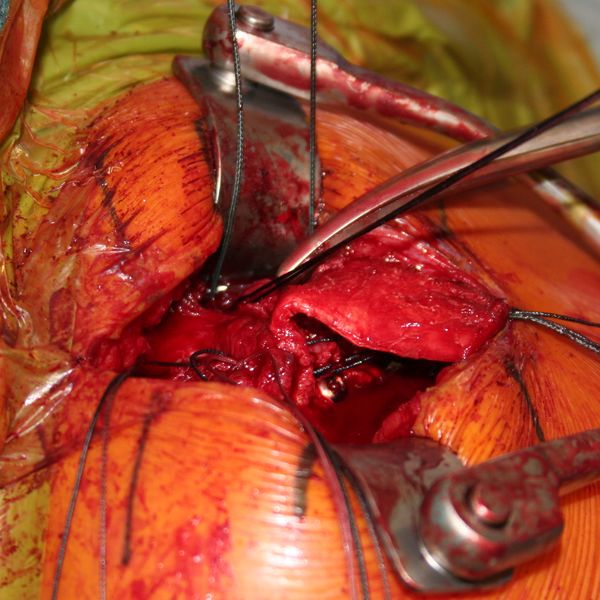
Allograft Reconstruction with Calcaneum and Achilles Tendon for an Irreparable Massive Rotator Cuff Tear with Bony Deficiency of the Greater Tuberosity (Video). Under general anesthesia and interscalene nerve block, the patient is placed in the beach chair position, with the operative arm draped free. An open anterosuperior incision with a deltoid split is performed in order to expose the greater tuberosity defect. The long head of the biceps was already resected. The remaining posterosuperior rotator cuff was carefully dissected and the proximal humeral head is debrided. The quality of cuff tissue is usually poor (Figure).
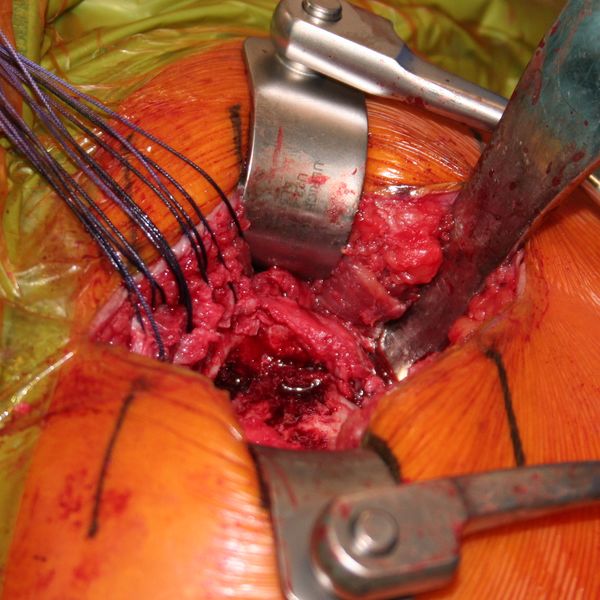
The Achilles tendon allograft with attached calcaneus is then prepared (Figure and Video).
The calcaneus is shaped to fill proximal humeral head defect. If necessary, the Achilles tendon is then split longitudinally to decrease its thickness, with the deep layer used to reinforce the rotator cuff repair. Then, the bony portion of the allograft is secured to the humeral head with a 4 mm malleolar screw under fluoroscopic control (Figure).
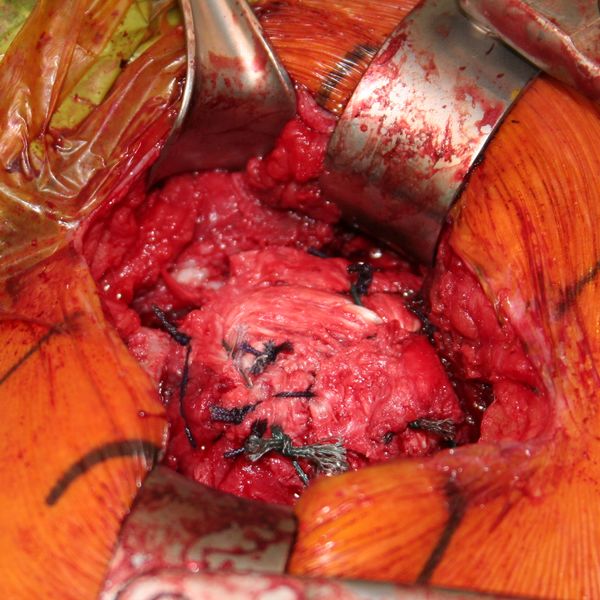
The native rotator cuff was then repaired onto the bony graft with a combination of an anchor and bone tunnels in the graft. The deep split of Achilles tendon is then sewn into the native posterosuperior rotator cuff to reinforce the repair (Figures and Video).
Two years follow-up confirm bony and tendinous integration (Figures).
Postoperatively, the patient wears an abduction pillow for six weeks and is allowed to perform pendulum exercises. After six weeks the abduction pillow is discontinued and passive mobilization is allowed. Full activity return and strengthening was permitted at three months, with a progressive increase of loads.
Type B: Full Thickness Tendon Lesion
B1: Lateral tendinous disruption
Full thickness disruption of the lateral tendon stump is the most frequent type of rotator cuff lesion, comprising approximately 90.1% of all surgically treated lesions (Table). Tendinous lesions most commonly involve the posterosuperior cuff. Subscapularis tears are nevertheless found in 59% of arthroscopic rotator cuff repairs.41 However, such tears are only full-thickness in 8.7% of cases, and are rarely isolated (Table).
Size of Tendon Lesion
Classifications for tear size include measurement in centimeters or number of tendons involved. This information can be derived from arthroscopy or magnetic resonance imaging and used to offer guidance on treatment and prognosis.[41][42][43][44][45]
Once size is identified and if massive, it can be further classified according to Collin et al.[46]
Tendon Retraction
Patte devised a method of classifying tendon coronal retraction that is often used for research purposes.[47]
The retraction is due to tendon and muscle shortening that are not synchronous after tendon tear.[48]
Substance loss in the later stages of musculotendinous retraction may be because of either active shortening of the tendon substance, suggesting that overreduction and lateral transposition of the tendon over the greater tuberosity may be unphysiological.
Tear Pattern
Full-thickness posterosuperior tears come in a variety of patterns. The most common categories include crescent tears, L and reverse L-shaped tears, and U-shaped tears accounting for respectively 40%, 30% and 15% of posterosuperior rotator cuff lesions.[49]
Recognition of these tear patterns is most useful for anatomic restoration during repair. Crescent tears have good medial to lateral mobility and are amenable to a double-row repair. Longitudinal tears (L and reverse L-shaped tears, and U-shaped tears) have greater mobility in 1 plane and typically require margin convergence to achieve complete repair. Finally, massive contracted tears have also been described. These tears have limited medial to lateral and anterior to posterior mobility and typically require advanced mobilization techniques (i.e. interval slides) to achieve repair.
Releases for the Rotator Cuff
In clinical practice, rotator cuff tears may present with a wide spectrum of size and mobility. Many massive rotator cuff tears may be reparable without releases and, in contrast, some small rotator cuff tears may require releases. Release is only required if tear may not be reduced to the footprint anatomically as it would otherwise unnecessarily increase the complexity of the procedure. Releases may be divided into bursal sided releases, articular sided releases (i.e. capsular release) and interval slides.
Double-Row Versus Single-Row Cuff Repair
Biomechanical testing has consistently demonstrated the superiority of double-row constructs.[50]
Within the domain of level I mid-term and short-term studies, double-row repair (Video) showed significant better UCLA score only (American Shoulder and Elbow Surgeons (ASES), Constant, WORC, and SANE scores showed no significance). This may correlate weakly with the significant lower partial-thickness retear rates of double-row repairs. In contrary, long-term level III studies showed a direct correlation of both functional outcomes and cuff structural integrity, with significant superiority of double-row over single-row repair techniques.[51]
Margin Convergence
Margin convergence to bone can be used in L or V tears. This technique accomplishes margin convergence between the two leaves of the cuff, and at the same time it anchors the cuff to bone, providing very secure fixation. Margin convergence to bone has the mechanical strain reduction advantage of margin convergence coupled with strong fixation to bone. This provides a very secure component to the overall fixation construct.
Load Sharing Rip Stop Construct
Double- row repair is not possible in the setting of medially based tears, lateral tendon loss, or limited tendon mobility. Load sharing rip stop constructs demonstrated improved functional outcomes with reasonable healing rates in an otherwise challenging subset of rotator cuff tears.[52]
This suture technique combines the advantages of a rip stop suture tape and load sharing properties of a double-row repair and has biomechanically superior properties compared to a single-row repair (Figure and Video).[53]
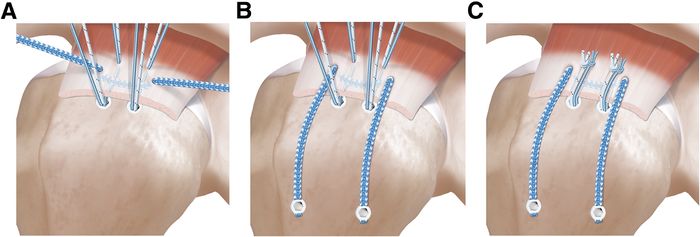
Massive Posterosuperior Rotator Cuff Tears
Prevalence
Massive rotator cuff tears comprise approximately 20% of all cuff tears and 80% of recurrent tears.[54][55]
Definition and Classification of Massive Rotator Cuff Tears
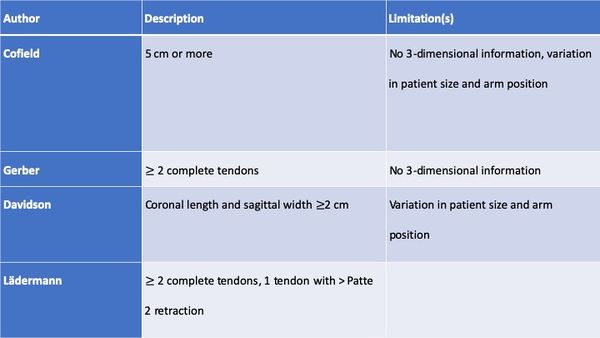
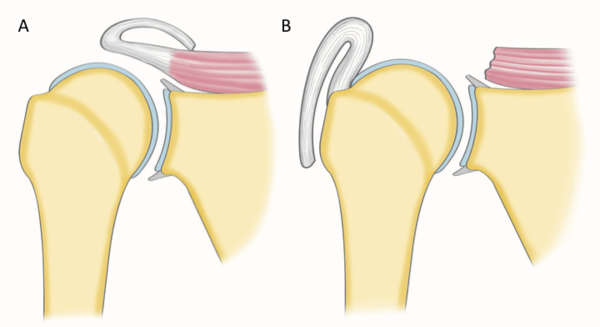
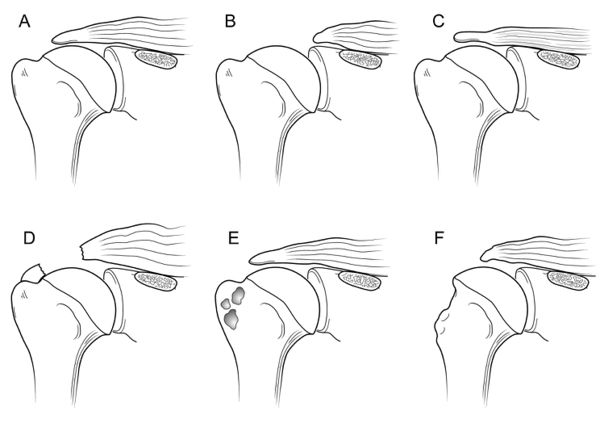
Historically a massive rotator cuff tear has been described as a tear with a diameter of 5 cm or more as described by Cofield or as a complete tear of two or more tendons as described by Gerber (Figure).[56][57]
The former in particular is usually applied at the time of surgery. In an attempt to provide a preoperative MRI-based classification, Davidson et al. defined a massive tear as one with a coronal length and sagittal width greater than or equal to 2 cm.[58]
Unfortunately, these systems are vulnerable to error due to variation in patient size and arm position at the time of measurement. It is more appropriate to define the size of a tear in terms of the amount of tendon that has been detached from the tuberosities. While the Gerber definition helps account for variability in size, there are exceptions to the complete two tendons requirement and this classification does not distinguish different patterns or predict function. Additionally, in using the term “massive”, there is a connotation of difficulty and irreparability. While challenging, most massive rotator cuff tears are reparable and other factors like the tendon retraction, atrophy, arthritis, and mobilization must be considered. Thus, in addition to the number of tendons involved, some authors proposed at least one of the two tendons must be retracted beyond the top of the humeral head (i.e Patte 3 for the supraspinatus in the coronal plane).[59]
Such classification also takes advantage of 3-dimensional information on tear pattern, providing guidance on treatment technique.
Table: Different Classification of Massive Rotator Cuff Tears
Once a massive rotator cuff tear is identified, it can be further classified according to Collin et al.2 In this subclassification, the rotator cuff is divided into five components: supraspinatus, superior subscapularis, inferior subscapularis, infraspinatus, and teres minor (Figure).
Rotator cuff tear patterns can then be classified into 5 types: type A, supraspinatus and superior subscapularis tears; type B, supraspinatus and entire subscapularis tears; type C, supraspinatus, superior subscapularis, and infraspinatus tears; type D, supraspinatus and infraspinatus tears; and type E, supraspinatus, infraspinatus, and teres minor tears (Figure).[60]
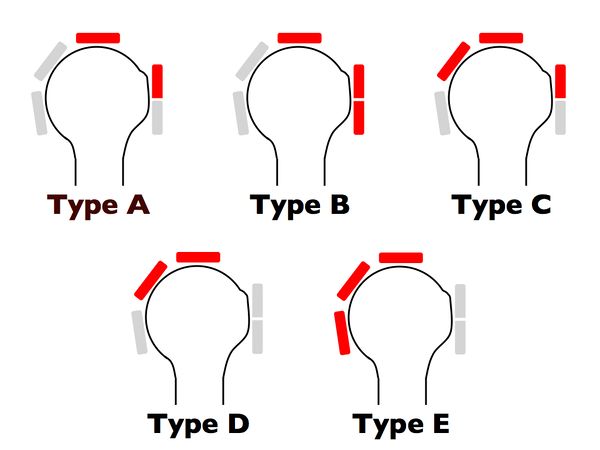
This classification not only subclassifies massive tears but has also been linked to function, particularly the maintenance of active elevation, offering more information than the traditional six sagittal segments of Patte’s classification.
Suprascapular Nerve Neuropathy and Massive Rotator Cuff Tear
Recently there has been growing interest in the relationship between suprascapular neuropathy and massive rotator cuff tears. Theoretically, medial retraction of posterosuperior rotator cuff tears can place excessive traction on the suprascapular nerve.[61]
However, clinical diagnosis is beset with uncertainties as the potential symptoms of suprascapular nerve neuropathy, namely, pain, weakness, and atrophy, are inseparable from those of massive rotator cuff tear. There is actually no support for routine suprascapular nerve release when repair is performed for several reasons. First, it is clearly demonstrated that repair of repair without release leads to satisfactory results. Moreover, the prevalence of suprascapular nerve neuropathy in case of massive rotator cuff tears in a prospective study is low (2%).[62]
Treatment Options for Massive Rotator Cuffs
It should be remembered that nonoperative treatment is successful in many cases. When surgery is indicated, the primary aim is restoration of force couples and anatomic or partial repair of the rotator cuff to its footprint. However, a number of factors (refusal of the patient, biologic factors, characteristics of the tear, etc) can make these goals difficult, impossible, or unwanted to achieve. Fatty infiltration, rotator cuff retraction, and poor tendon compliance are common in patients with massive rotator cuff tears. In these situations, other approaches have been advocated, with varying degrees of success.[63]
These include physical therapy,59,60 subacromial decompression and palliative biceps tenotomy (subacromial debridement),61 muscle transfer,62 and reverse shoulder arthroplasty.63 However, there are no randomized controlled trials comparing these various options and recommendations are mainly based on retrospective case series and the surgeon’s own experiences.[64][65][66][67][68]
Conservative Treatment
Many patients with massive rotator cuff tears respond favorably to nonsurgical treatment. Nevertheless, patients must be aware that despite clinical improvement, future treatment may be impacted by progression of glenohumeral osteoarthritis and fatty infiltration as well as narrowing of the acromiohumeral distance. In a series of 19 patients with massive rotator cuff tears treated nonoperatively the average Constant score was 83% at a mean follow-up of 48 months. However, 50% of “reparable” tears became “irreparable” during this period.[69]
The mainstay of nonoperative treatment includes nonsteroidal anti-inflammatory drugs, subacromial corticosteroid injections, and physical therapy. The protocol of rehabilitation focused habitually on global deltoid reconditioning and periscapular strengthening. Although certain authors proposed that re-education of the anterior deltoid muscle to compensate for a deficient rotator cuff is the cornerstone, we attach more importance to solicitation of stabilizing muscles of the glenohumeral joint with an approach based on exercises in high position. In this position, the deltoid, which acts synergistically with the remaining rotator muscles, has no upward component and participates in the articular coaptation.[70]
In general, nonoperative management is attempted for six months before considering surgery. Younger patients (<60 years of age), however, may be immediate candidates for surgery based on the high risk for progression with conservative treatment. If after six months, symptoms have not improved, the chances of success with further nonoperative treatment decreases and operative treatment may be considered for older patients. It is unclear if it is exercise alone or exercise in combination with other interventions during the recovery process that offers the greatest benefit. In a recent prospective cohort of 45 patients suffering from pseudoparalysis with a radiographically confirmed massive rotator cuff tears, Collin and al. found after a follow-up of 48 months that the mean Constant score improved from 43 to 56 points and the mean forward flexion improved from 76 degrees to more than 160 degrees after completion of the program. They also demonstrated that effectiveness of physical therapy is related to the size and location of the lesion; if the tear involved the posterosuperior rotator cuff (B type), or only two tendons or less, most patients regained active anterior elevation that persisted for 48 months. The anterior rotator cuff is the key of anterior active elevation as only 20% of patients with MRCTs, but an intact subscapularis, develop pseudoparalysis.
Surgical (Operative) Treatment
For older patients surgery is considered when nonoperative treatment fails. Additionally, we often consider surgery as first line treatment in young patients because there is a high rate of progression with conservative treatment and for tears involving the anterior rotator cable since this area is most important to maintenance of forward elevation.
Arthroscopic Rotator Cuff Repair
The approach is to repair all of the rotator cuff that can reasonably be brought back to the tuberosities without excessive tension, and to address all potential causes of persistent pain or factors threatening the repair. The goal of a repair, even if partial, is to restore force couples and to re-establish the “suspension bridge”. In this theory, complete closure of the defect is less important than restoration of a stable fulcrum for normal shoulder kinematics. Although shoulder strength may not improve after this intervention, function is usually enhanced because of relief from pain caused by mechanical impingement. Additionally, although complete healing of massive tears is not always achievable, we believe that partial healing of the cuff may prevent secondary extension of the tear.
The Acromion and Biceps
Complete anterior acromioplasty is not advisable in the setting of a massive tear as it may lead to postoperative anterosuperior migration of the humeral head. The acromiohumeral arch is probably a component of human evolution used to compensate the deficiency of the superior rotator cuff.66 Consequently, when and how should acromioplasty should be perform is still debated.[71]
A tenotomy or tenodesis of the long head of the biceps should be perform in the setting of a massive rotator cuff tear. There is evidence suggesting that the long head of the biceps tendon may be a source of pain and contributes to the discomfort. In a large series, Walch et al. observed an increase in the Constant score from 48.4 preoperatively to 67.6 after arthroscopic biceps tenotomy. At last follow-up, 87% of patients were satisfied or very satisfied with the result. However, the acromiohumeral interval decreased by a mean of 1.3 mm during the follow-up period.[72]
Repair Techniques
Unfortunately, even if reinsertion of the tendon on the bone is achievable, it is often difficult to reliably achieve long-term healing with a structurally intact repair.[73]
In the setting of a massive tear, a double-row repair improves long-term functional outcome.68-70[74][75][76]
However, this should not be performed at the expense of over-tensioning as application of a double-row repair to a tendon with poor tendon length and excursion may lead to medial failure.[77]
Augmentation
Graft augmentation may improve healing in massive rotator cuff tears, but add significant cost and time to the procedure.[78]
The choice of graft is influenced by several factors including mechanical properties, host response and potential for ingrowth. Scaffolds provide mechanical support and have biological properties that may favorably influence cell proliferation and differentiation, hopefully improving tendon-to-bone healing. Currently, scaffolds derived from dermis, small intestinal submucosa, skin, fascia lata, and pericardium have been processed and marketed for augmentation in the repair of massive tears. Biological grafts are preferred, when compared to synthetic grafts, due to the unknown host response to synthetic grafts. An important factor in the longevity and strength of a graft is the amount of ingrowth.
Results of Primary Repairs
Results following arthroscopic repair of massive rotator cuffs have previously been reported.[79][80]
For primary repair, improvements are observed in forward flexion (132 degrees vs 168 degrees), pain (6.3 v 1.3), UCLA score, (15.7 v 30.7) and American Shoulder and Elbow Surgeons score (41.7 v 85.7) (P<.001). A good or excellent outcome is obtained in 78% of cases. Similar results are noticed after repair of type A, B and C massive rotator cuff tears.[81]
After revision repair, mean active forward elevation improves by 15 degrees, from 136.0 degrees ± 51.9 degrees (range, 30 to 180 degrees) at baseline to 151.4 degrees ± 41.5 degrees (range, 30 to 180 degrees) at final follow-up (P=.019). The mean pain score improves by 3.1 points, from 5.0 ± 2.4 points at baseline to 1.9 ± 2.3 points at final follow-up (p<.001). The mean American Shoulder and Elbow Surgeons (ASES) score improves from 45.7 ± 17.8 at baseline to 75.5 ± 20.3 at final follow-up (P<.001). The mean UCLA score also improves, from 16.7 ±4.9 at baseline to 26.4 ± 6.9 at final follow-up (P<.001). According to the UCLA score, functional results are excellent in 15% of cases, good in 35%, fair in 25%, and poor in 25%. Seventy-nine percent of the patients are satisfied, and 32 patients (60%) returned to their previous activities.[82]
Revision Rotator Cuff Repair
Introduction
Failure of tendon healing after rotator cuff repair is common, reported in approximately 20% of cases depending on tear size.[83]
Tear recurrence can be related to various factors such as: 1) inadequate strength of the initial repair construct, 2) biological failure to heal despite strong initial fixation, and 3) inappropriate post-operative rehabilitation causing structural failure of the repair.[84][85][86][87]
While functional outcome has been correlated with postoperative rotator cuff integrity78 many patients maintain a satisfactory outcome despite structural failure.[88]
The ideal treatment for a recurrent tear is thus not completely defined.
Initial Radiological Findings
The goal of imaging studies is to confirm the site of the recurrent tear. Trantalis et al. reported five patients with retearing of the cuff after double-row rotator cuff repair. All five patients had retearing medial to the medial row if sutures were placed near the musculotendinous junction of the supraspinatus.[89]
Hayashida et al. observed that the prevalence of complete retearing of the tendon after a double-row rotator cuff repair is similar around the medial anchors with a well-preserved footprint.[90]
Another point of interest is the quality of the tendon.[91]
A significant and growing number of rotator cuff repairs are performed in individuals with poor rotator cuff tissue quality. Djurasovic reported an incidence of 30% (24 on 80) of poor rotator cuff tissue quality (graded subjectively at the time of surgery).[92]
At the same time, the muscle undergoes intrinsic degeneration. After a retear, Deniz et al. found that fatty infiltration and atrophy continued to worsen significantly.[93]
However, fatty infiltration of the supraspinatus does not seem to be a determinant factor in tendon healing. Park et al. did not find significant relationship between preoperative supraspinatus fatty infiltration and postoperative tendon healing. Contrarily, it seems that fatty infiltration of infraspinatus and subscapularis are highly significant factors (P<.001).[94]
Another point is the bone quality. Oh et al. demonstrated that bone mineral density within the greater tuberosity decreases in patients with rotator cuff tears. In another retrospective study that investigated the relationship between greater tuberosity osteopenia and chronicity of rotator cuff tears, Cadet et al. found that there were significantly greater osteopenic changes in the greater tuberosity in patients with chronic retracted rotator cuff tears.[95]
However, this localized osteoporosis may not influence tendon healing. In a recent study, Park et al. did not observe after primary repair that bone mineral density influenced final results.[96]
Nevertheless, the greater tuberosity in revision cases can also be deficient due to anchor removal or perianchor cyst formation. Kim et al. observed in a retrospective case series of two hundred and nine patients bone cyst formation in 97 instances (46.4%), and these authors questioned the utility of bioabsorbable anchors because of possible interference with revision surgery. Consequent bone lysis can be noticed after trauma. Lädermann et al. reported massive bone resorption after osteosynthesis of the greater tuberosity leading to combined tendon and bony insufficiency and pseudoparalysis/pseudoparesis.
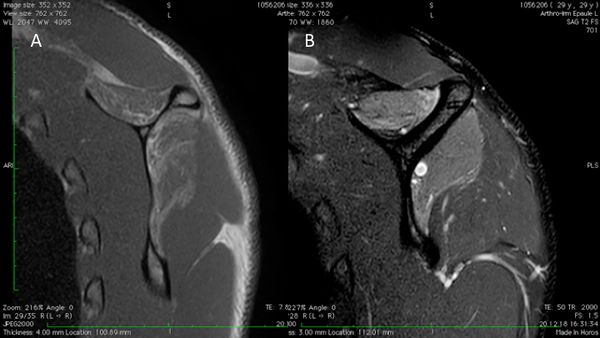
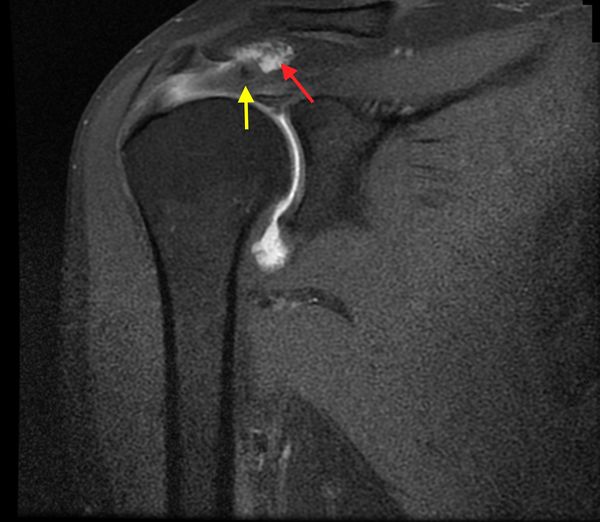
When milestones of typical post-operative recovery are not met, analysis of rotator cuff repair should be considered and a multi-modal evaluation is required. The goal of imaging studies is to confirm the site of the recurrent tear (Figure)86,92 the type of failure (e.g. in continuity), and if possible, it’s cause.[97][98][99]
Other points of interest are the quality of the bone (tuberosity deficiency), tendon, and muscle, and whether further surgery is feasible.[100][101]
Standard shoulder radiographs, including anteroposterior, axillary lateral and scapular Y (outlet) views, may demonstrate decreased acromiohumeral distance, glenohumeral arthritis, subacromial spurs, acetabularization of the acromion, femoralization of the humeral head, and implant or anchor migration.[102][103]
It can also be used to rule out chondrolysis, anchor migration or prominence, and acromial fracture. Among evaluation techniques, the most widely accepted reference standard is magnetic resonance imaging which allows visualization of the tendons and does not involve radiation exposure. Intra-articular contrast may be used in association with magnetic resonance imaging to increase the sensitivity for detecting a recurrent tear.[104]
Postoperative magnetic resonance imaging images are difficult to interpret; inadequate coverage of the greater or lesser tuberosity may indicate partial healing and not a recurrent full-thickness tear.[105][106][107]
Furthermore, only 10% of re-attached tendons generate a normal magnetic resonance imaging signal. Thus, a common finding is the presence of an intermediate signal within the tendon indicating granulation tissue or of a low-intensity signal produced by fibrous tissue.[108][109]
These signal changes may persist for longer than six months, due to tissue remodeling, and seem to have no clinical implications.[110][111]
Finally, the evaluation of magnetic resonance imaging scans is made difficult by the normal leakage of fluid into the subacromial space after the opening of the rotator interval and passage of instruments through the tendon, which may contain artifacts generated, for instance, by metal anchors or high-strength sutures. These factors, together with the high cost of magnetic resonance imaging, lend considerable appeal to ultrasound as a method for evaluating rotator cuff repair, even if its effectiveness is operator-dependent.[112]
Computed tomography arthrogram can also be used to aid in the identification of recurrent rotator cuff tears when neither ultrasound or magnetic resonance imaging are options.[113]
Failure after rotator cuff repair was previously believed to occur during the first three months.[114][115]
While the majority of retears do occur within the first three months, it has now been demonstrated retears can occur up to six months after repair.[116][117][118]
Recent prospective studies have confirmed that ultrasound has a high sensitivity and specificity for detecting a recurrent rotator cuff tear compared to magnetic resonance imaging.In a study comparing magnetic resonance imaging and ultrasound after rotator cuff repair, Codsi et al. found 92% agreement with a coefficient of 0.70.[119]
Similarly, Collin et al. reported that ultrasound had 80% sensitivity and 98% specificity compared to magnetic resonance imaging.[120]
Treatment
Non Surgical (Conservative) Treatment of Failed Rotator Cuff Repair
Jost et al. evaluated 20 patients with a failed rotator cuff repair at a mean follow-up of 38 months and reported that the adjusted Constant score and shoulder simple value averaged 83% and 75%, respectively.[121]
Namdari et al. demonstrated a successful outcome in 54% of patients (defined by an American Shoulder and Elbow Surgeons score of more than 80 points) and a mean 15 point improvement in the American Shoulder and Elbow Surgeons score at a mean of 52 months postoperative. Finally, the same group compared the two and ten year results for patients with known structural failures of rotator cuff repair. The average long-term American Shoulder and Elbow Surgeons score was 79 points (range, 50 to 95 points) and the average visual analog scale pain score was 2.2 points (range, 1 to 4 points); both scores were unchanged from those at two years. The average simple shoulder test score was 9.2 points (range, 6 to 12 points), and the average age-adjusted Constant score was 73 points (range, 59 to 90 points).[122]
Surgical (Operative) Treatment Revision Rotator Cuff Repair
Surgical Technique
The alarming retear rate indicates that several surgical options can be considered which must be individualized to the patient. For example, in the setting of an acute traumatic retear in a physiologically young, healthy, active and non-pseudoparalytic patient, arthroscopic revision surgery is generally recommended. Techniques to enhance mechanical fixation, such as linked load-sharing rip-stop constructs should be considered.[123]
Augmented repair using scaffold devices derived from autografts,46,119,120 allograft,121 xenograft extracellular matrix122 or synthetic matrices such as poly-l-lactide grafts123 have been used to offer a structural support of the repair during the crucial healing period and to improve healing rates. The scientific literature does not contain enough data to justify any systematic associated augmentation techniques. Tendon transfers may be used in patients without advanced glenohumeral arthritis who have significant loss of external rotation strength and maintain anterior active elevation.62,124 If the patient is young, pseudoparalytic and suffers from a combined bony and tendinous rotator cuff insufficiency, calcaneum and Achilles tendon allograft could be considered.40
Finally, whereas primary pseudoparalysis responds well to arthroscopic rotator cuff repair, persistent pseudoparalysis after a previous attempt at rotator cuff repair may be more predictably managed with reverse shoulder arthroplasty. Denard et al. reported that pseudoparalysis/pseudoparesis was reversed in the revision setting in only 43% of patients with a low rate (54%) of satisfaction.[124]
In contrast, Boileau et al. found that anterior elevation was reliably restored with reverse shoulder arthroplasty after failed rotator cuff repair and 73% of patients were satisfied.[125]
Clinical and Radiological Results After Revision Rotator Cuff Repair
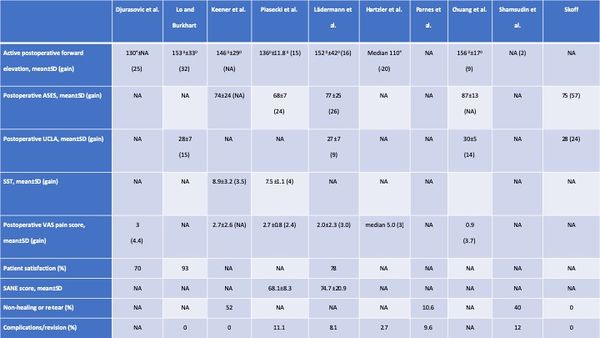
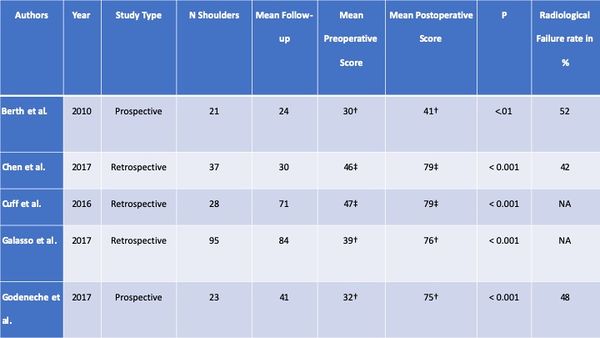
The clinical results of are summarized in the Table. Overall, range of motion improved, except in one series of open rotator cuff repair.94 Functional outcome improved in all series and 70% or more of patients were satisfied or very satisfied.[126]
Table: Clinical Results of Revision RCR
Complication
The short- to intermediate-term incidence of complications, including subsequent revision surgery, after revision rotator cuff repair is relatively low, around ten percent in this review (Table). However, most studies primarily considered reoperation a complication and did not examine complications such as hematoma, hardware failure, and postoperative stiffness. The prevalence of postoperative complications is therefore probably higher than reported. The prevalence of non-healing or retear was around 40% (range, 0 to 62%) in the four studies with postoperative imaging.[127][128][129][130]
Furthermore, these tears may progress with time; Shamsudin et al. reported a prevalence of defect of 28% at six months and of 40% at two years. If revision is planned, patients have to be aware of the high prevalence of persistent structural defect. Moreover, retear rate after reoperation continues to deteriorate with time.[131][132]
Structural failure does not always result in clinical failure. Many patients with partial healing of the cuff and a residual defect will be much improved after surgery. Characteristics associated with successful and unsuccessful results after structural failure of rotator cuff repair are poorly understood. Retear or non-healing of tendons is rather frequent and surgery is rarely proposed because this condition is often well tolerated with marked clinical improvement in comparison with the preoperative state.[133]
One reason for clinical failure is probably the non-restoration of balanced force couples and the suspension bridge system of force transmission in the shoulder. The location (involvement of the subscapularis on which the rotator cable is attached) and the size (more than two tendons) are the primary determinant of rotator cuff function.
Risk Factors for Postoperative Poorer Results
Several patient-related factors appear to be associated with poorer results. The most important factor seems to be poor preoperative range of motion. Female sex and, in one study, if the surgery was performed on the dominant arm, were negatively associated with postoperative outcome.[134][135][136]
There is still controversy about certain risk factors such as age of patients. Disease-related factors included patients with a recurrent tear after the revision repair, preoperative visual analogue scale pain score greater than five, and poor preoperative range of motion. The range vary from less than 90 degrees in the studies from Denard et al. and Piasecki et al. to 140 degrees in the study of Chuang et al.[137][138][139]
The latter factor has been reported in almost all series and is probably the most important preoperative indicator. In addition, acromiohumeral distance (less than seven mm) can be associated with a satisfactory outcome.[140]
There is controversy about patients with more than one prior surgery, with one study reporting that this negatively impacted results and another study reporting that it did not.[141][142]
Operative-related factors like poor tendon quality54,88 is associated with poorer results.[143][144]
One study compared outcomes between massive and non-massive tears and did not find any significant difference in terms of post-operative anterior elevation, pain, or functional outcome.[145]
Post-revision Rehabilitation
In all studies, subjects took part in standardized rehabilitation protocols. Most studies did not allow immediate overhead passive motion. Most studies recommend sling during 6 weeks. Strengthening is delayed until six to sixteen weeks post-operatively. Full return to activity was not allowed until four to twelve months.
B2: Medial Tendinous Disruption
Disruption of the tendon medial to an intact lateral tendon stump has been reported in primary chronic and acute cases or postoperatively as a failure medial to the medial row.[146][147]
Regarding the former, it is now understood that the infraspinatus insertion is quite broad and wraps around from posterior to anterior to occupy much of the lateral greater tuberosity. Therefore, such descriptions of a lateral tendon stump remaining may, in fact, represent a torn supraspinatus with an intact infraspinatus.
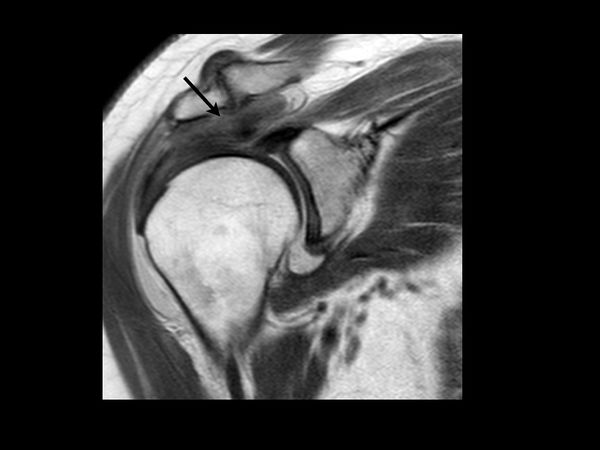
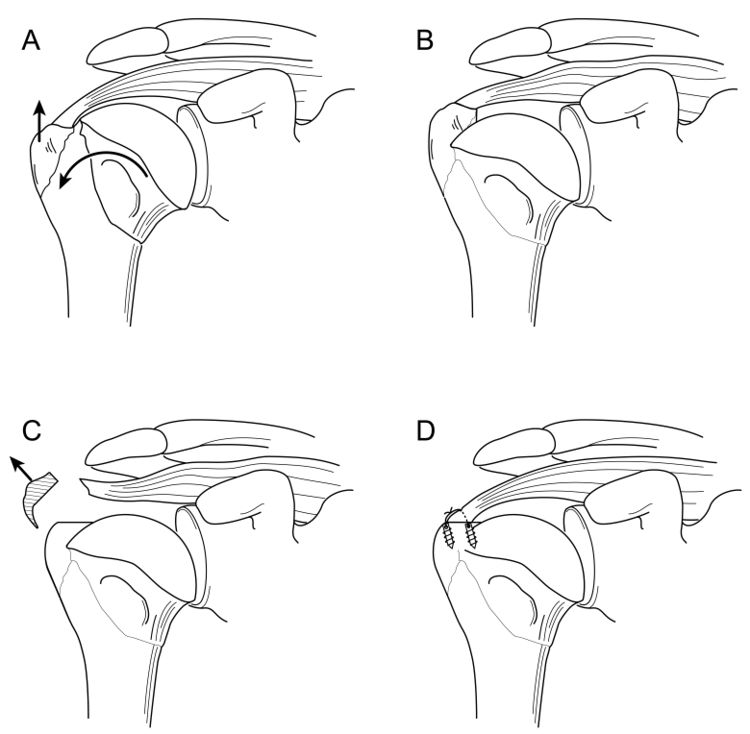
Full thickness defects medial to an intact footprint of the rotator cuff can be seen following a rotator cuff repair (Figure). Trantalis et al. described 5 patients with medial failure following a double-row rotator cuff repair. Such failure results from overtensioning during repair and is very difficult to manage with revision repair. These lesions do not produce muscular edema, except in traumatic cases with important and acute retraction of the muscle and the remnant of the tendon (Figure); its origin is then either retraction (which may appear in some hours) or neurological lesions (noted after some weeks).[148]
B3: Tendon to Tendon Adhesion: “Fosbury Flop Tear”
The Fosbury flop tear occurs from a full thickness tear that has flipped upon itself and adhered medially (Figure).[149]
Prevalence
In a prospective study spanning one-year, Lädermann et al. reported five patients with full or partial-thickness rotator cuff lesions in a series of 97 (5 % incidence rate).142 Radiologically, these lesions showed a thicker than normal tendon stump on the bursal-side of the retracted supraspinatus tendon in a superomedial orientation.[150]
Additionally, patients with this lesion were also found to have an accumulation of fluid in the superomedial part of the subacromial bursa as well as adhesions between the wall of the subacromial bursa and the tendon of the supraspinatus. Since the original description, another group verified the same entity.
151. Nakamizo H. Arthroscopic repair for subacromial incarceration of a torn rotator cuff. AP-SMART 2015;2:90-4.
Surgical Technique
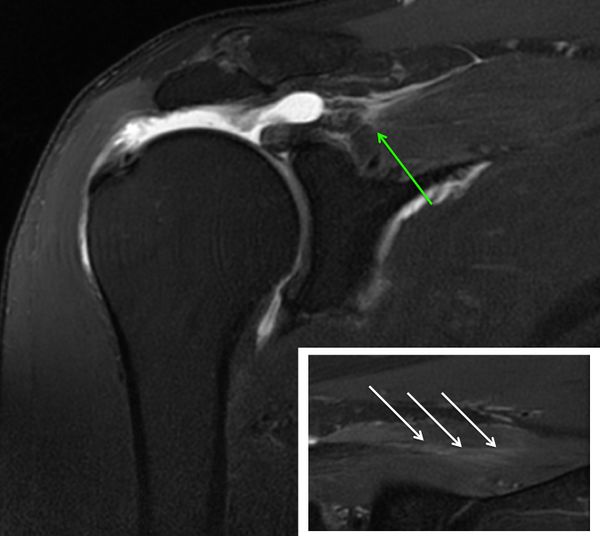
A diagnostic arthroscopy is performed with an arthroscopic pump maintaining pressure at 50 mm Hg. The biceps is tenotomized or tenodesed. Attention is then turned to the posterosuperior rotator cuff (supraspinatus and infraspinatus tendons). The appearances of the tears are unusual with ulcerations on the bursal surface (anemone like) and what initially appeared to be a thickened lateral tendon stump (Figure and Video).
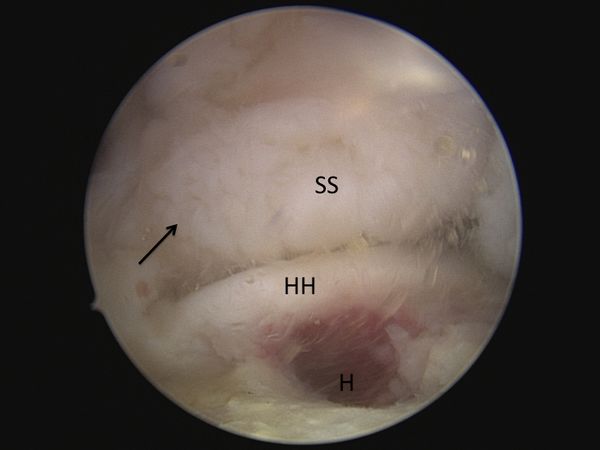
After a progressive dissection, however, medial adhesions of the bursal layer is found. A complete excavation of the rotator cuff must be performed by skeletonizing the scapular spine medially, removing any bursal leaders (false insertions into the internal deltoid fascia) laterally, and debriding the fibrofatty bursa overlying the rotator cuff. These steps allow identification of the lateral tendon stump which is reversed upon itself and scarred medially. Once identified the tendon stump is unfolded (Video) and subsequently repaired to the lateral bone bed.
B4: Tendon to Acromion Adhesion
Disruption of the lateral tendon stump can be followed by adhesion under the acromion, the coracoid process or the coracoacromial arch (Figure). These adhesions are most pronounced in revision situations, but may also be observed in primary cases, particularly in the setting of a massive contracted rotator cuff tear.[151]

Type C: Musculotendinous Junction Lesion
Isolated ruptures of the musculotendinous junction are rare in the rotator cuff, but have a dramatic impact on functional outcome. Such lesions have been observed in all muscles of the rotator cuff, affecting the infraspinatus muscle in half of cases, followed by the supraspinatus in 31% of cases, the subscapularis in 25%, and the teres minor in 19% of cases (more than one muscle is occasionally involved).[152]
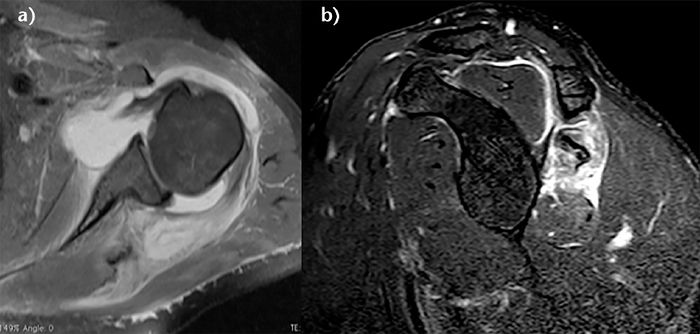
These lesions can been classified into 3 stages. Grade I injuries are a muscular strain that heals without adverse sequelae. Grade II injuries are partial ruptures without tendon retraction. Grade III injuries are complete ruptures at the musculotendinous junction.146 The acute phase of these injuries are associated with severe inflammation, leading to a highly characteristic bright signal on T2 weighted magnetic resonance imaging (Figure).[153]
Edema of rotator cuff muscle with an intact tendon-bone insertion is infrequent. It has been described in cases of denervation, such as compression of the suprascapular nerve, Parsonage Turner syndrome, in other rare and non-specific conditions.[154][155]
Complete musculotendinous junction ruptures have only been described in the infraspinatus and the supraspinatus.[156][157][158]
Possible causes for musculotendinous junction infraspinatus lesion are calcific tendinitis or previous cortisone injection. On the other hand, rupture of the other muscles seem to be due to trauma or inlet impingement syndrome. There is little information about the clinical results of grade 3 musculotendinous junction lesions.
Type C with Reverse Fosbury Flop Tear
Exceptionally, a lesion of the musculotendinous junction can develop a Fosbury pattern and heals, for its tendon part, on the humerus or even coracoid process.[159]

Type D: Muscle Insufficiency
D1: Fatty Infiltration and Muscle Atrophy
One of the most important prognostic factor for rotator cuff repair is nonfunctional muscle bellies. Muscle quality is most commonly classified according to Goutallier et al. to determine the extent of injuries based upon the degree in which fat is present in the muscle. They proposed a 5 stage classification system of fatty infiltration. Additionally, they demonstrated that multiple muscles develop fatty degeneration, even if they were not directly impacted by the original lesion.[160]
With the advent of MRI, however, the classification was extrapolated to the most lateral parasagittal image on which the scapular spine was in contact with the scapular body (Y view).[161]
The mean time to tendon rupture observed for stage 2 fatty infiltration is 3 years for the supraspinatus and 2.5 years for the infraspinatus and the subscapularis when their tendons ruptured. The mean time observed to grade 3 and 4 fatty infiltration is 5, 4, and 3 years for the supraspinatus, the infraspinatus, and the subscapularis, respectively.[162]
Zanetti et al. described a radiographic tangent sign to quickly and reliably assess the presence or absence of supraspinatus atrophy on MRI. This sign is a reliable method for evaluating the presence or absence of muscle atrophy using the sagittal plane and is moreover significantly related to the level of fatty infiltration within the supraspinatus muscle.[163]
It has been reported to be a predictor of whether a rotator cuff tear will be repairable.[164]
Thomazeau et al. proposed calculating the occupation ratio of the supraspinatus muscle belly using MRI. This was calculating by comparing the supraspinatus fossa volume to total supraspinatus muscle belly volume and computing the ratio. This ratio was found to be significantly decreased in patients with repairable rotator cuff tears.[165]
Inability to obtain a complete repair of the supraspinatus was associated with a positive tangent sign (30% irreparable) versus a negative tangent sign (6.3% irreparable, OR = 6,3, P =0.0102) and with Goutallier grade 3-4 fatty infiltration of the supraspinatus (42.9% irreparable) versus grade 0-2 fatty infiltration (5.7% irreparable, OR = 11.8, P =0.001).[166]
Rotator cuff repair should thus be performed before the appearance of fatty infiltration (Stage 2) and atrophy (positive tangent sign) and as soon as possible in older patients when the tear involves multiple tendons.[167]
D2: Neurological impairment
Isolated suprascapular nerve neuropathy is a condition associated with acute and chronic shoulder girdle traction injuries, compressive lesions such as paralabral cysts and compressive ligaments, as well as large or massive rotator cuff tears (Figure). In the latter situation, the proposed mechanism involves traction of the nerve caused by retraction of the supraspinatus against its fixed points on the suprascapular and spinoglenoid notches. However, clinical diagnosis is beset with uncertainties as the potential symptoms of suprascapular nerve neuropathy, namely, pain, weakness, and atrophy, are inseparable from those of rotator cuff tear. Currently, there is no support for routine suprascapular nerve release as the prevalence of suprascapular nerve neuropathy in the setting of a massive rotator cuff tear was very low (2%) in a recent prospective study.[168]
D3: Tumors
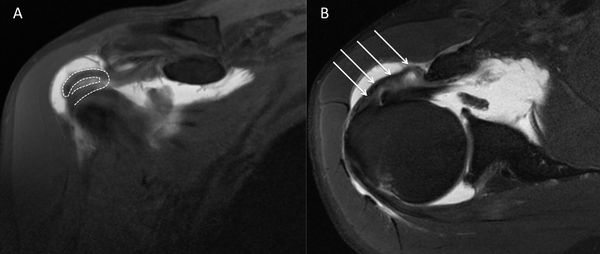
Numerous tumors, such as an arthrosynovial cyst, intramuscular lipoma, or a calcified hematoma, can developed at the expense of the muscular tissue and cause muscular insufficiency (Figure).[169]
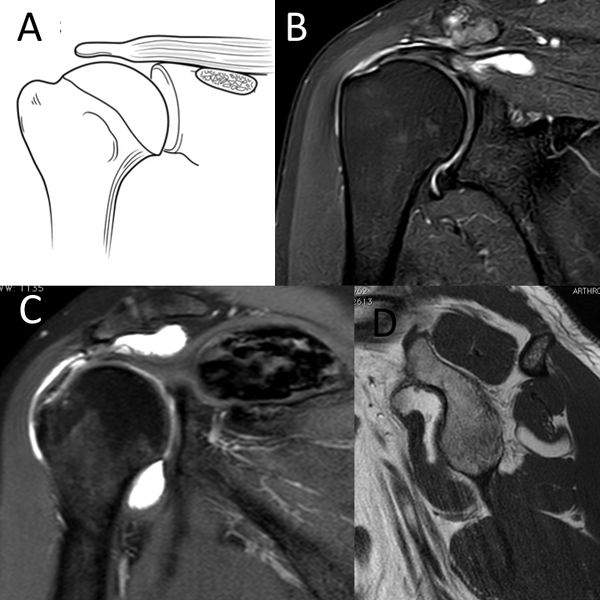
C) Coronal T2-weighted PD D) sagittal T1-weighted image demonstrating D3 rotator cuff lesion with a calcified hematoma in the supraspinatus and an intramuscular lipoma of the subscapularis, respectively. Reproduced from Lädermann et al., with permission.
These impairments can be as isolated or be associated with other rotator cuff lesions. Management may require treatment of the associated mechanical stress in addition to rotator cuff repair.
Irreparable Rotator Cuff Tears
Introduction
One of the most challenging issues in shoulder surgery is the management of symptomatic irreparable rotator cuff tears. The literature reports that 12% of posterosuperior rotator cuff tear are not repairable.[170]
The latter condition when symptomatic can be managed with several approaches without clear evidence based guidelines. For example, the same patient with a D type irreparable rotator cuff tears according to Collin et al. may be offered physiotherapy, partial repair, tendon transfer, superior capsular reconstruction, subacromial spacer (balloon), or even a reverse shoulder arthroplasty (RSA) depending on multiple factors, including, geography, surgeon experience, implants costs, etc. Moreover, even if it is reported that these surgical procedures have different indications, they are often applied to patients with similar problems indiscriminately.
Definition of an Irreparable Rotator Cuff Tears & Clinical and Imaging Findings
The definition of an irreparable rotator cuff varies widely. Furthermore, with advances in anchors, suture strength, techniques of release and repair with load-sharing rip-stop fixation, etc, the definition continues to evolve. Two situations can be faced; the first one consists of a patient who has a contra-indication to cuff repair, and the second scenario is intra-operative when a complete repair is not physically possible. While most rotator cuff tears can be repaired, some lesions are not reparable or should not be repaired. Imaging studies play a critical role in preoperative assessment, evaluation of the defects and selection of the correct treatment for an irreparable rotator cuff tears. The following clinical and radiological preoperative factors that have been clearly associated with postoperative clinical or radiological failure should be considered before attempting repair.
Clinical Examination
Pseudoparalysis was defined as a chronic inability to actively elevate the arm beyond 90 degrees with full passive forward flexion.[171]
It is nevertheless important to note that this correspond to functional limitation associated or not with an antero-superior escape and not just to pain inhibition. Several studies purport to reverse pseudoparalysis although it represents mainly pseudoparesis cases. When pain inhibition or slight stiffness limits the patient from elevating the shoulder, the limited motion is not secondary to complete cuff deficiency.[172]
Anatomically, pseudoparalysis requires the disruption of at least of rotator cable attachment which in the study of Collin et al. was found in only 2.9% of massive D-type cases. This means that pseudoparalysis of the posterosuperior rotator cuff cases involved usually the whole posterior cuff (33.3% of pseudoparalysis found in E-type irreparable rotator cuff tears).[173]
In addition to pseudoparalysis, the presence of lag signs (external rotation lag, drop, dropping, hornblower signs) is also associated with non-reparability.[174]
Treatment
Non-Surgical (Conservative) Treatment
As patients with posterosuperior irreparable rotator cuff tears do not have anterosuperior escape, many respond favorably to nonsurgical treatment which should be attempted for six months before considering surgery. If after this adequate period of time symptoms have not improved, the chances of success with further non-operative treatment decreases and operative treatment may be considered. The mainstay of non-operative treatment includes nonsteroidal anti-inflammatory drugs, subacromial corticosteroid injections, and physical therapy. Levy et al. prospectively assessed 17 patients with clinically and radiographically diagnosed irreparable rotator cuff tears that underwent an anterior deltoid training program.[175]
By 9 months, the mean Constant score improved from 26 to 63, and the forward flexion improved from 40° to 160°. In another prospective cohort of 45 patients suffering from pseudoparalysis with a radiographically confirmed D-type rotator cuff tear, Collin et al. found after a follow-up of 48 months that 14 of 15 patients had substantial improvement in active forward elevation to above 90 degrees.[176]
The protocol of rehabilitation focused habitually on a multimodal physical therapy program with global deltoid reconditioning and periscapular strengthening.[177]
Certain authors propose that reeducation of the anterior deltoid muscle to compensate for a deficient rotator cuff is the cornerstone of successful non-operative treatment.[178]
The promising results has nevertheless not been confirmed.[179][180]
Surgical (Operative) Treatment
In the absence of a gold standard surgical solution, treatment of irreparable rotator cuff tears has proven to be quite challenging, adding to the surgeon’s dilemma regarding the choice of patient and treatment option. Younger active patients (<60 years of age) with traumatic tears, may be immediate candidates for surgery based on the high risk for progression with conservative treatment. Surgical approaches have been advocated, with varying degrees of success. The surgical options include arthroscopic debridement, partial repair, biceps procedure, superior capsular reconstruction, muscle transfers, biodegradable subacromial spacer interposition, biological augmentation and reverse shoulder arthroplasty. Despite all these options, irreparable rotator cuff tears are difficult to manage and treat effectively. There are no high levels of evidence prospective trials comparing these various options and therefore recommendations are mainly based on retrospective case series, surgeon experiences, and expert opinions.
Long Head of the Biceps Tenotomy or Tenodesis +/- Partial Repair
This procedure includes biceps tenotomy or tenodesis, partial repair if evaluation has deemed the remaining tendon to be of good quality, and associated procedures such as distal clavicle resection if necessary. Tenotomy or tenodesis of the long head of the biceps should consistently be performed, as biceps tendinopathy is observed in 92% of rotator cuff lesions.[181]
There is evidence suggesting that this structure is a source of pain and contributes to the symptomatology of patients with irreparable rotator cuff tears. Walch et al. reported statistically significant improvements in the Constant score with an isolated biceps tenotomy (Constant score 48 points preoperatively to 68 points at follow-up (P < .0001)) which has been confirmed by numerous authors.[182]
The aim of this procedure is to repair all of the rotator cuff tendon that can reasonably be brought back to the tuberosities without excessive tension, and to address all potential causes of persistent pain or factors threatening the repair. The goal of a partial repair is to restore force couples, to re-establish the “suspension bridge”, and to prevent secondary extension of the tear. In this theory, complete closure of the defect is less important than restoration of a stable fulcrum for normal shoulder kinematics. Although having little effect on improvement in shoulder strength after this intervention, eliminating various pain generators usually enhances function. Although a partial cuff repair is conducted, the role of the biceps tenotomy should not be overlooked in the patient improvements observed.[183]
Acromioplasty is not advisable in the setting of an irreparable rotator cuff tears as it may lead to postoperative antero-superior migration of the humeral head. Tuberoplasty has been proposed as an alternative to classic subacromial decompression in order to preserve the integrity of the coracoacromial arch.[184]
Although the results in compensated tears and low-demand patients are promising,176 it is currently unknown if the positive effect with regards to pain relief is due to the tuberoplasty or to the concomitant performed bursectomy, synovectomy and biceps treatment. Partial repair provides good clinical outcomes, comparable to those reported with biceps sacrifice and subacromial decompression. The main purported benefit of repairing part of the cuff is its potential to slow or halt further tear progression and to increase the strength of the shoulder. All series of partial repair reported a significant improvement in functional scores, while reporting a rate of radiological repair failure around 50% (Table).[185][186][187][188][189][190][191][192]
Long-term benefit in prevention of head migration has not been demonstrated.
Table: Results of partial repair of irreparable rotator cuff tear.
References
- ↑ Burkhart SS, Nottage WM, Ogilvie-Harris DJ, Kohn HS, Pachelli A. Partial repair of irreparable rotator cuff tears. Arthroscopy 1994;10:363-70.
- ↑ Collin P, Matsumura N, Lädermann A, Denard PJ, Walch G. Relationship between massive chronic rotator cuff tear pattern and loss of active shoulder range of motion. J Shoulder Elbow Surg 2014;23:1195-202.
- ↑ Denard PJ, Lädermann A, Brady PC, et al. Pseudoparalysis From a Massive Rotator Cuff Tear Is Reliably Reversed With an Arthroscopic Rotator Cuff Repair in Patients Without Preoperative Glenohumeral Arthritis. Am J Sports Med 2015;43:2373-8.
- ↑ Jobe FW, Moynes DR. Delineation of diagnostic criteria and a rehabilitation program for rotator cuff injuries. Am J Sports Med 1982;10:336-9.
- ↑ Chalmers PN, Cvetanovich GL, Kupfer N, et al. The champagne toast position isolates the supraspinatus better than the Jobe test: an electromyographic study of shoulder physical examination tests. J Shoulder Elbow Surg 2016;25:322-9.
- ↑ Gerber C, Blumenthal S, Curt A, Werner CM. Effect of selective experimental suprascapular nerve block on abduction and external rotation strength of the shoulder. J Shoulder Elbow Surg 2007;16:815-20.
- ↑ Hertel R, Ballmer FT, Lombert SM, Gerber C. Lag signs in the diagnosis of rotator cuff rupture. J Shoulder Elbow Surg 1996;5:307-13.
- ↑ Collin P, Treseder T, Denard PJ, Neyton L, Walch G, Lädermann A. What is the Best Clinical Test for Assessment of the Teres Minor in Massive Rotator Cuff Tears? Clin Orthop Relat Res 2015;473:2959-66.
- ↑ Patte D, Goutallier D. [Grande libération antérieure dans l'épaule douloureuse par conflit antérieur]. Rev Chir Orthop Reparatrice Appar Mot 1988;74:306-11.
- ↑ Walch G, Boulahia A, Calderone S, Robinson AH. The 'dropping' and 'hornblower's' signs in evaluation of rotator-cuff tears. J Bone Joint Surg Br 1998;80:624-8.
- ↑ Neer C. Cuff tears, biceps lesions, and impingement. In: Neer C, ed. Shoulder reconstruction. Philadelphia: W. B. Saunders Company; 1990:41-142.
- ↑ Walch G, Boulahia A, Calderone S, Robinson AH. The 'dropping' and 'hornblower's' signs in evaluation of rotator-cuff tears. J Bone Joint Surg Br 1998;80:624-8.
- ↑ Nove-Josserand L, Edwards TB, O'Connor DP, Walch G. The acromiohumeral and coracohumeral intervals are abnormal in rotator cuff tears with muscular fatty degeneration. Clin Orthop Relat Res 2005:90-6.
- ↑ Werner CM, Conrad SJ, Meyer DC, Keller A, Hodler J, Gerber C. Intermethod agreement and interobserver correlation of radiologic acromiohumeral distance measurements. J Shoulder Elbow Surg 2008;17:237-40.
- ↑ Sheean AJ, Hartzler RU, Denard PJ, et al. Preoperative Radiographic Risk Factors for Incomplete Arthroscopic Supraspinatus Tendon Repair in Massive Rotator Cuff Tears. Arthroscopy. 2018 Apr;34(4):1121-1127.
- ↑ Hamid N, Omid R, Yamaguchi K, Steger-May K, Stobbs G, Keener JD. Relationship of radiographic acromial characteristics and rotator cuff disease: a prospective investigation of clinical, radiographic, and sonographic findings. J Shoulder Elbow Surg 2012;21:1289-98.
- ↑ Zanca P. Shoulder pain: involvement of the acromioclavicular joint. (Analysis of 1,000 cases). Am J Roentgenol Radium Ther Nucl Med 1971;112:493-506.
- ↑ Plomb-Holmes C, Clavert P, Kolo F, Tay E, Ladermann A, French Society of A. An orthopaedic surgeon's guide to ultrasound imaging of the healthy, pathological and postoperative shoulder.Orthop Traumatol Surg Res. 2018 Dec;104(8S):S219-S232.
- ↑ Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med 2007;35:719-28.
- ↑ Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res 1994:78-83.
- ↑ Sheean AJ, Hartzler RU, Denard PJ, et al. Preoperative Radiographic Risk Factors for Incomplete Arthroscopic Supraspinatus Tendon Repair in Massive Rotator Cuff Tears. Arthroscopy. 2018 Apr;34(4):1121-1127.
- ↑ Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med 2012;40:606-10.
- ↑ Zanetti M, Gerber C, Hodler J. Quantitative assessment of the muscles of the rotator cuff with magnetic resonance imaging. Investigative radiology 1998;33:163-70.
- ↑ Williams MD, Lädermann A, Melis B, Barthelemy R, Walch G. Fatty infiltration of the supraspinatus: a reliability study. J Shoulder Elbow Surg 2009;18:581-7.
- ↑ Kissenberth MJ, Rulewicz GJ, Hamilton SC, Bruch HE, Hawkins RJ. A positive tangent sign predicts the repairability of rotator cuff tears. J Shoulder Elbow Surg 2014;23:1023-7.
- ↑ Sheean AJ, Hartzler RU, Denard PJ, et al. Preoperative Radiographic Risk Factors for Incomplete Arthroscopic Supraspinatus Tendon Repair in Massive Rotator Cuff Tears. Arthroscopy. 2018 Apr;34(4):1121-1127.
- ↑ Thomazeau H, Rolland Y, Lucas C, Duval JM, Langlais F. Atrophy of the supraspinatus belly. Assessment by MRI in 55 patients with rotator cuff pathology. Acta Orthop Scand 1996;67:264-8.
- ↑ Niglis L, Dosch JC. Intra- and inter-observer agreement in MRI assessment of rotator cuff healing using the Sugaya, Goutallier, Warner and Thomazeau classifications 10 years after surgery. “s.l.” and “s.n.”: Université de Strasbourg; 2015.
- ↑ Court-Brown CM, Garg A, McQueen MM. The epidemiology of proximal humeral fractures. Acta Orthop Scand 2001;72:365-71.
- ↑ Neer CS, 2nd. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am 1970;52:1077-89.
- ↑ Bono CM, Renard R, Levine RG, Levy AS. Effect of displacement of fractures of the greater tuberosity on the mechanics of the shoulder. J Bone Joint Surg Br 2001;83:1056-62.
- ↑ Greiner S, Scheibel M. [Bony avulsions of the rotator cuff : Arthroscopic concepts]. Der Orthopade 2011;40:21-4, 6-30.
- ↑ Burkhart SS, Klein JR. Arthroscopic repair of rotator cuff tears associated with large bone cysts of the proximal humerus: compaction bone grafting technique. Arthroscopy 2005;21:1149.
- ↑ Lädermann A, Denard PJ, Burkhart SS. Arthroscopic management of proximal humerus malunion with tuberoplasty and rotator cuff retensioning. Arthroscopy 2012;28:1220-9.
- ↑ Burkhart SS, Klein JR. Arthroscopic repair of rotator cuff tears associated with large bone cysts of the proximal humerus: compaction bone grafting technique. Arthroscopy 2005;21:1149. Complete reabsorption of the tuberosities have also been observed (Figure).
- ↑ Moore DR, Cain EL, Schwartz ML, Clancy WG, Jr. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med 2006;34:392-6.
- ↑ Roche CP, Diep P, Hamilton M, et al. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bulletin of the Hospital for Joint Disease 2013;71:284-93.
- ↑ Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg 2013;22:1199-208.
- ↑ Sershon RA, Van Thiel GS, Lin EC, et al. Clinical outcomes of reverse total shoulder arthroplasty in patients aged younger than 60 years. J Shoulder Elbow Surg 2014;23:395-400.
- ↑ Lädermann A, Denard P, Abrassart S, Schwitzguébel A. Achilles Tendon Allograft for an Irreparable Massive Rotator Cuff Tear with Bony Deficiency of the Greater Tuberosity: A Case Report. Knee Surg Sports Traumatol Arthrosc 2016;Jan 25.
- ↑ Cofield RH. Rotator cuff disease of the shoulder. J Bone Joint Surg Am 1985;67:974-9.
- ↑ Davidson J, Burkhart SS. The geometric classification of rotator cuff tears: a system linking tear pattern to treatment and prognosis. Arthroscopy 2010;26:417-24.
- ↑ Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am 2000;82:505-15.
- ↑ Harryman DT, 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA, 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am 1991;73:982-9.
- ↑ Lädermann A, Denard PJ, Collin P. Massive rotator cuff tears: definition and treatment. International orthopaedics 2015;39:2403-14.
- ↑ Collin P, Matsumura N, Lädermann A, Denard PJ, Walch G. Relationship between massive chronic rotator cuff tear pattern and loss of active shoulder range of motion. J Shoulder Elbow Surg 2014;23:1195-202.
- ↑ Patte D. Classification of rotator cuff lesions. Clin Orthop Relat Res 1990:81-6.
- ↑ Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med 2012;40:606-10.
- ↑ Davidson J, Burkhart SS. The geometric classification of rotator cuff tears: a system linking tear pattern to treatment and prognosis. Arthroscopy 2010;26:417-24.
- ↑ Hohmann E, König A, Kat CJ, Glatt V, Tetsworth K, Keough N.Single- versus double-row repair for full-thickness rotator cuff tears using suture anchors. A systematic review and meta-analysis of basic biomechanical studies. Eur J Orthop Surg Traumatol. 2018 Jul;28(5):859-868.
- ↑ Sobhy MH, Khater AH, Hassan MR, El Shazly O. Do functional outcomes and cuff integrity correlate after single- versus double-row rotator cuff repair? A systematic review and meta-analysis study. Eur J Orthop Surg Traumatol. 2018 May;28(4):593-605.
- ↑ Noyes MP, Lädermann A, Denard PJ. Functional Outcome and Healing of Large and Massive Rotator Cuff Tears Repaired With a Load-Sharing Rip-Stop Construct. Arthroscopy. 2017 Sep;33(9):1654-1658.
- ↑ Denard PJ, Burkhart SS. A load-sharing rip-stop fixation construct for arthroscopic rotator cuff repair. Arthrosc Tech 2012;1:e37-42.
- ↑ Lo IK, Burkhart SS. Arthroscopic revision of failed rotator cuff repairs: technique and results. Arthroscopy 2004;20:250-67.
- ↑ Burkhart SS, Danaceau SM, Pearce CE, Jr. Arthroscopic rotator cuff repair: Analysis of results by tear size and by repair technique-margin convergence versus direct tendon-to-bone repair. Arthroscopy 2001;17:905-12.
- ↑ Cofield RH. Subscapular muscle transposition for repair of chronic rotator cuff tears. Surg Gynecol Obstet 1982;154:667-72.
- ↑ Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am 2000;82:505-15.
- ↑ Davidson J, Burkhart SS. The geometric classification of rotator cuff tears: a system linking tear pattern to treatment and prognosis. Arthroscopy 2010;26:417-24.
- ↑ Lädermann A, Denard PJ, Collin P. Massive rotator cuff tears: definition and treatment. International orthopaedics 2015;39:2403-14.
- ↑ Collin P, Matsumura N, Lädermann A, Denard PJ, Walch G. Relationship between massive chronic rotator cuff tear pattern and loss of active shoulder range of motion. J Shoulder Elbow Surg 2014;23:1195-202.
- ↑ Albritton MJ, Graham RD, Richards RS, 2nd, Basamania CJ. An anatomic study of the effects on the suprascapular nerve due to retraction of the supraspinatus muscle after a rotator cuff tear. J Shoulder Elbow Surg 2003;12:497-500.
- ↑ Collin P, Treseder T, Lädermann A, et al. Neuropathy of the suprascapular nerve and massive rotator cuff tears: a prospective electromyographic study. J Shoulder Elbow Surg 2014;23:28-34.
- ↑ Berhouet J, Collin P, Benkalfate T, et al. Massive rotator cuff tears in patients younger than 65 years. Epidemiology and characteristics. Orthopaedics & traumatology, surgery & research : OTSR 2009;95:S13-8.
- ↑ Collin PG, Gain S, Nguyen Huu F, Lädermann A. Is rehabilitation effective in massive rotator cuff tears? Orthopaedics & traumatology, surgery & research : OTSR 2015;101:S203-5.
- ↑ Zingg PO, Jost B, Sukthankar A, Buhler M, Pfirrmann CW, Gerber C. Clinical and structural outcomes of nonoperative management of massive rotator cuff tears. J Bone Joint Surg Am 2007;89:1928-34.
- ↑ Walch G, Edwards TB, Boulahia A, Nove-Josserand L, Neyton L, Szabo I. Arthroscopic tenotomy of the long head of the biceps in the treatment of rotator cuff tears: clinical and radiographic results of 307 cases. J Shoulder Elbow Surg 2005;14:238-46.
- ↑ Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am 2013;95:1920-6.
- ↑ Wall B, Nove-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am 2007;89:1476-85.
- ↑ Zingg PO, Jost B, Sukthankar A, Buhler M, Pfirrmann CW, Gerber C. Clinical and structural outcomes of nonoperative management of massive rotator cuff tears. J Bone Joint Surg Am 2007;89:1928-34.
- ↑ Collin PG, Gain S, Nguyen Huu F, Lädermann A. Is rehabilitation effective in massive rotator cuff tears? Orthopaedics & traumatology, surgery & research : OTSR 2015;101:S203-5.
- ↑ Voisin JL, Ropars M, Thomazeau H. The human acromion viewed from an evolutionary perspective. Orthopaedics & traumatology, surgery & research : OTSR 2014;100:S355-60.
- ↑ Walch G, Edwards TB, Boulahia A, Nove-Josserand L, Neyton L, Szabo I. Arthroscopic tenotomy of the long head of the biceps in the treatment of rotator cuff tears: clinical and radiographic results of 307 cases. J Shoulder Elbow Surg 2005;14:238-46.
- ↑ Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am 2008;90:2423-31.
- ↑ Carbonel I, Martinez AA, Calvo A, Ripalda J, Herrera A. Single-row versus double-row arthroscopic repair in the treatment of rotator cuff tears: a prospective randomized clinical study. Int Orthop 2012;36:1877-83.
- ↑ Connelly TM, Shaw A, O'Grady P. Outcome of open massive rotator cuff repairs with double-row suture knotless anchors: case series. Inter Orthop 2015.
- ↑ Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of arthroscopic massive rotator cuff repair: the importance of double-row fixation. Arthroscopy 2012;28:909-15.
- ↑ Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med 2012;40:606-10.
- ↑ Barber FA, Burns JP, Deutsch A, Labbe MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy 2012;28:8-15.
- ↑ Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy 2012;28:1214-9.
- ↑ Lädermann A, Denard PJ, Burkhart SS. Midterm outcome of arthroscopic revision repair of massive and nonmassive rotator cuff tears. Arthroscopy 2011;27:1620-7.
- ↑ Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of arthroscopic massive rotator cuff repair: the importance of double-row fixation. Arthroscopy 2012;28:909-15.
- ↑ Lädermann A, Denard PJ, Burkhart SS. Midterm outcome of arthroscopic revision repair of massive and nonmassive rotator cuff tears. Arthroscopy 2011;27:1620-7.
- ↑ Collin P, Abdullah A, Kherad O, Gain S, Denard PJ, Lädermann A. Prospective evaluation of clinical and radiologic factors predicting return to activity within 6 months after arthroscopic rotator cuff repair. J Shoulder Elbow Surg 2015;24:439-45.
- ↑ Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am 2005;87:1229-40.
- ↑ Cho NS, Moon SC, Jeon JW, Rhee YG. The influence of diabetes mellitus on clinical and structural outcomes after arthroscopic rotator cuff repair. Am J Sports Med 2015;43:991-7.
- ↑ Chung SW, Oh JH, Gong HS, Kim JY, Kim SH. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med 2011;39:2099-107.
- ↑ Clement ND, Hallett A, MacDonald D, Howie C, McBirnie J. Does diabetes affect outcome after arthroscopic repair of the rotator cuff? J Bone Joint Surg Br 2010;92:1112-7.
- ↑ Jost B, Zumstein M, Pfirrmann CW, Gerber C. Long-Term Outcome After Structural Failure of Rotator Cuff Repairs. J Bone Joint Surg Am 2006;88:472-9.
- ↑ Trantalis JN, Boorman RS, Pletsch K, Lo IK. Medial rotator cuff failure after arthroscopic double-row rotator cuff repair. Arthroscopy 2008;24:727-31.
- ↑ Hayashida K, Tanaka M, Koizumi K, Kakiuchi M. Characteristic retear patterns assessed by magnetic resonance imaging after arthroscopic double-row rotator cuff repair. Arthroscopy 2012;28:458-64.
- ↑ Denard PJ, Burkhart SS. Techniques for managing poor quality tissue and bone during arthroscopic rotator cuff repair. Arthroscopy 2011;27:1409-21.
- ↑ Djurasovic M, Marra G, Arroyo JS, Pollock RG, Flatow EL, Bigliani LU. Revision rotator cuff repair: factors influencing results. J Bone Joint Surg Am 2001;83-A:1849-55.
- ↑ Deniz G, Kose O, Tugay A, Guler F, Turan A. Fatty degeneration and atrophy of the rotator cuff muscles after arthroscopic repair: does it improve, halt or deteriorate? Arch Orthop Trauma Surg 2014;134:985-90.
- ↑ Park JS, Park HJ, Kim SH, Oh JH. Prognostic Factors Affecting Rotator Cuff Healing After Arthroscopic Repair in Small to Medium-sized Tears. Am J Sports Med 2015;43:2386-92.
- ↑ Cadet ER, Hsu JW, Levine WN, Bigliani LU, Ahmad CS. The relationship between greater tuberosity osteopenia and the chronicity of rotator cuff tears. J Shoulder Elbow Surg 2008;17:73-7.
- ↑ Park JS, Park HJ, Kim SH, Oh JH. Prognostic Factors Affecting Rotator Cuff Healing After Arthroscopic Repair in Small to Medium-sized Tears. Am J Sports Med 2015;43:2386-92.
- ↑ Hayashida K, Tanaka M, Koizumi K, Kakiuchi M. Characteristic retear patterns assessed by magnetic resonance imaging after arthroscopic double-row rotator cuff repair. Arthroscopy 2012;28:458-64.
- ↑ Mellado JM, Calmet J, Olona M, et al. MR assessment of the repaired rotator cuff: prevalence, size, location, and clinical relevance of tendon rerupture. European radiology 2006;16:2186-96.
- ↑ cCarron JA, Derwin KA, Bey MJ, et al. Failure with continuity in rotator cuff repair "healing". Am J Sports Med 2013;41:134-41.
- ↑ Lädermann A, Denard P, Abrassart S, Schwitzguébel A. Achilles Tendon Allograft for an Irreparable Massive Rotator Cuff Tear with Bony Deficiency of the Greater Tuberosity: A Case Report. Knee Surg Sports Traumatol Arthrosc 2016;Jan 25.
- ↑ Deniz G, Kose O, Tugay A, Guler F, Turan A. Fatty degeneration and atrophy of the rotator cuff muscles after arthroscopic repair: does it improve, halt or deteriorate? Arch Orthop Trauma Surg 2014;134:985-90.
- ↑ Hartzler RU, Sperling JW, Schleck CD, Cofield RH. Clinical and radiographic factors influencing the results of revision rotator cuff repair. International journal of shoulder surgery 2013;7:41-5.
- ↑ Nove-Josserand L, Edwards TB, O'Connor DP, Walch G. The acromiohumeral and coracohumeral intervals are abnormal in rotator cuff tears with muscular fatty degeneration. Clin Orthop Relat Res 2005:90-6.
- ↑ de Jesus JO, Parker L, Frangos AJ, Nazarian LN. Accuracy of MRI, MR arthrography, and ultrasound in the diagnosis of rotator cuff tears: a meta-analysis. AJR American journal of roentgenology 2009;192:1701-7.
- ↑ Khazzam M, Kuhn JE, Mulligan E, et al. Magnetic resonance imaging identification of rotator cuff retears after repair: interobserver and intraobserver agreement. Am J Sports Med 2012;40:1722-7.
- ↑ Saccomanno MF, Cazzato G, Fodale M, Sircana G, Milano G. Magnetic resonance imaging criteria for the assessment of the rotator cuff after repair: a systematic review. Knee Surg Sports Traumatol Arthrosc 2015;23:423-42.
- ↑ Motamedi AR, Urrea LH, Hancock RE, Hawkins RJ, Ho C. Accuracy of magnetic resonance imaging in determining the presence and size of recurrent rotator cuff tears. J Shoulder Elbow Surg 2002;11:6-10.
- ↑ Khazzam M, Kuhn JE, Mulligan E, et al. Magnetic resonance imaging identification of rotator cuff retears after repair: interobserver and intraobserver agreement. Am J Sports Med 2012;40:1722-7.
- ↑ Zanetti M, Hodler J. MR imaging of the shoulder after surgery. Radiologic clinics of North America 2006;44:537-51, viii.
- ↑ Tudisco C, Bisicchia S, Savarese E, et al. Single-row vs. double-row arthroscopic rotator cuff repair: clinical and 3 Tesla MR arthrography results. BMC musculoskeletal disorders 2013;14:43.
- ↑ Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am 1999;81:1281-90.
- ↑ Ok JH, Kim YS, Kim JM, Yoo TW. Learning curve of office-based ultrasonography for rotator cuff tendons tears. Knee Surg Sports Traumatol Arthrosc 2013;21:1593-7.
- ↑ Nazarian LN, Jacobson JA, Benson CB, et al. Imaging algorithms for evaluating suspected rotator cuff disease: Society of Radiologists in Ultrasound consensus conference statement. Radiology 2013;267:589-95.
- ↑ Kluger R, Bock P, Mittlbock M, Krampla W, Engel A. Long-term survivorship of rotator cuff repairs using ultrasound and magnetic resonance imaging analysis. Am J Sports Med 2011;39:2071-81.
- ↑ Miller BS, Downie BK, Kohen RB, et al. When do rotator cuff repairs fail? Serial ultrasound examination after arthroscopic repair of large and massive rotator cuff tears. Am J Sports Med 2011;39:2064-70.
- ↑ Barth J, Fotiadis E, Barthelemy R, Genna S, Saffarini M. Ultrasonic evaluation of the repair integrity can predict functional outcomes after arthroscopic double-row rotator cuff repair. Knee Surg Sports Traumatol Arthrosc 2015;23:376-85.
- ↑ Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am 2013;95:965-71.
- ↑ Kim JH, Hong IT, Ryu KJ, Bong ST, Lee YS, Kim JH. Retear rate in the late postoperative period after arthroscopic rotator cuff repair. Am J Sports Med 2014;42:2606-13.
- ↑ Codsi MJ, Rodeo SA, Scalise JJ, Moorehead TM, Ma CB. Assessment of rotator cuff repair integrity using ultrasound and magnetic resonance imaging in a multicenter study. J Shoulder Elbow Surg 2014;23:1468-72.
- ↑ Collin P, Yoshida M, Delarue A, et al. Evaluating postoperative rotator cuff healing: Prospective comparison of MRI and ultrasound. Orthopaedics & traumatology, surgery & research : OTSR 2015;101:S265-8.
- ↑ Jost B, Zumstein M, Pfirrmann CW, Gerber C. Long-Term Outcome After Structural Failure of Rotator Cuff Repairs. J Bone Joint Surg Am 2006;88:472-9.
- ↑ Paxton ES, Teefey SA, Dahiya N, Keener JD, Yamaguchi K, Galatz LM. Clinical and radiographic outcomes of failed repairs of large or massive rotator cuff tears: minimum ten-year follow-up. J Bone Joint Surg Am 2013;95:627-32.
- ↑ Noyes MP, Lädermann A, Denard PJ. Functional Outcome and Healing of Large and Massive Rotator Cuff Tears Repaired With a Load-Sharing Rip-Stop Construct. Arthroscopy. 2017 Sep;33(9):1654-1658
- ↑ Denard PJ, Lädermann A, Brady PC, et al. Pseudoparalysis From a Massive Rotator Cuff Tear Is Reliably Reversed With an Arthroscopic Rotator Cuff Repair in Patients Without Preoperative Glenohumeral Arthritis. Am J Sports Med 2015;43:2373-8.
- ↑ Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg 2009;18:600-6.
- ↑ Hartzler RU, Sperling JW, Schleck CD, Cofield RH. Clinical and radiographic factors influencing the results of revision rotator cuff repair. International journal of shoulder surgery 2013;7:41-5.
- ↑ Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. International journal of shoulder surgery 2012;6:36-44.
- ↑ Lenart BA, Martens KA, Kearns KA, Gillespie RJ, Zoga AC, Williams GR. Treatment of massive and recurrent rotator cuff tears augmented with a poly-l-lactide graft, a preliminary study. J Shoulder Elbow Surg 2015;24:915-21.
- ↑ Keener JD, Wei AS, Kim HM, et al. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome. J Bone Joint Surg Am 2010;92:590-8.
- ↑ Shamsudin A, Lam PH, Peters K, Rubenis I, Hackett L, Murrell GA. Revision versus primary arthroscopic rotator cuff repair: a 2-year analysis of outcomes in 360 patients. Am J Sports Med 2015;43:557-64.
- ↑ Shamsudin A, Lam PH, Peters K, Rubenis I, Hackett L, Murrell GA. Revision versus primary arthroscopic rotator cuff repair: a 2-year analysis of outcomes in 360 patients. Am J Sports Med 2015;43:557-64.
- ↑ Vastamaki M, Lohman M, Borgmastars N. Rotator cuff integrity correlates with clinical and functional results at a minimum 16 years after open repair. Clin Orthop Relat Res 2013;471:554-61.
- ↑ Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am 2014;96:99-105.
- ↑ Chuang MJ, Jancosko J, Nottage WM. Clinical outcomes of single-row arthroscopic revision rotator cuff repair. Orthopedics 2014;37:e692-8.
- ↑ Piasecki DP, Verma NN, Nho SJ, et al. Outcomes after arthroscopic revision rotator cuff repair. Am J Sports Med 2010;38:40-6.
- ↑ Lädermann A, Denard PJ, Burkhart SS. Midterm outcome of arthroscopic revision repair of massive and nonmassive rotator cuff tears. Arthroscopy 2011;27:1620-7.
- ↑ Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy 2012;28:1214-9.
- ↑ Chuang MJ, Jancosko J, Nottage WM. Clinical outcomes of single-row arthroscopic revision rotator cuff repair. Orthopedics 2014;37:e692-8.
- ↑ Piasecki DP, Verma NN, Nho SJ, et al. Outcomes after arthroscopic revision rotator cuff repair. Am J Sports Med 2010;38:40-6.
- ↑ Hartzler RU, Sperling JW, Schleck CD, Cofield RH. Clinical and radiographic factors influencing the results of revision rotator cuff repair. International journal of shoulder surgery 2013;7:41-5.
- ↑ Lädermann A, Denard PJ, Burkhart SS. Midterm outcome of arthroscopic revision repair of massive and nonmassive rotator cuff tears. Arthroscopy 2011;27:1620-7.
- ↑ Piasecki DP, Verma NN, Nho SJ, et al. Outcomes after arthroscopic revision rotator cuff repair. Am J Sports Med 2010;38:40-6.
- ↑ Djurasovic M, Marra G, Arroyo JS, Pollock RG, Flatow EL, Bigliani LU. Revision rotator cuff repair: factors influencing results. J Bone Joint Surg Am 2001;83-A:1849-55.
- ↑ Lo IK, Burkhart SS. Arthroscopic revision of failed rotator cuff repairs: technique and results. Arthroscopy 2004;20:250-67.
- ↑ Lädermann A, Denard PJ, Burkhart SS. Midterm outcome of arthroscopic revision repair of massive and nonmassive rotator cuff tears. Arthroscopy 2011;27:1620-7.
- ↑ Trantalis JN, Boorman RS, Pletsch K, Lo IK. Medial rotator cuff failure after arthroscopic double-row rotator cuff repair. Arthroscopy 2008;24:727-31.
- ↑ Loew M, Magosch P, Lichtenberg S, Habermeyer P, Porschke F. How to discriminate between acute traumatic and chronic degenerative rotator cuff lesions: an analysis of specific criteria on radiography and magnetic resonance imaging. J Shoulder Elbow Surg 2015;24:1685-93.
- ↑ Fleckenstein JL, Watumull D, Conner KE, et al. Denervated human skeletal muscle: MR imaging evaluation. Radiology 1993;187:213-8.
- ↑ Lädermann A, Denard PJ, Kolo FC. A new tear pattern of the rotator cuff and its treatment: Fosbury flop tears. International journal of shoulder surgery 2015;9:9-12.
- ↑ Lädermann A, Denard PJ, Kolo FC. A new tear pattern of the rotator cuff and its treatment: Fosbury flop tears. International journal of shoulder surgery 2015;9:9-12.
- ↑ Romeo AA, Loutzenheiser T, Rhee YG, Sidles JA, Harryman DT, 2nd, Matsen FA, 3rd. The humeroscapular motion interface. Clin Orthop Relat Res 1998:120-7.
- ↑ Taneja AK, Kattapuram SV, Chang CY, Simeone FJ, Bredella MA, Torriani M. MRI findings of rotator cuff myotendinous junction injury. AJR American journal of roentgenology 2014;203:406-11.
- ↑ Zarins B, Ciullo JV. ACute muscle and tendon injuries in athletes. Clinics in sports medicine 1983;2:167-82.
- ↑ Ludig T, Walter F, Chapuis D, Mole D, Roland J, Blum A. MR imaging evaluation of suprascapular nerve entrapment. European radiology 2001;11:2161-9.
- ↑ Bredella MA, Tirman PF, Fritz RC, Wischer TK, Stork A, Genant HK. Denervation syndromes of the shoulder girdle: MR imaging with electrophysiologic correlation. Skeletal radiology 1999;28:567-72.
- ↑ Walch G, Nove-Josserand L, Liotard JP, Noel E. Musculotendinous infraspinatus ruptures: an overview. Orthopaedics & traumatology, surgery & research : OTSR 2009;95:463-70.
- ↑ Hertel R, Lambert SM. Supraspinatus rupture at the musculotendinous junction. J Shoulder Elbow Surg 1998;7:432-5.
- ↑ Lädermann A, Christophe FK, Denard PJ, Walch G. Supraspinatus rupture at the musclotendinous junction: an uncommonly recognized phenomenon. J Shoulder Elbow Surg 2012;21:72-6.
- ↑ Tirefort J, Cunningham G, Lädermann A. Reverse Fosbury Flop Tear of the Rotator Cuff. Case Reports in Orthopedics 2017;2017:3635897.
- ↑ Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res 1994:78-83.
- ↑ Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg 1999;8:599-605.
- ↑ Melis B, Nemoz C, Walch G. Muscle fatty infiltration in rotator cuff tears: descriptive analysis of 1688 cases. Orthopaedics & traumatology, surgery & research : OTSR 2009;95:319-24.
- ↑ Williams MD, Lädermann A, Melis B, Barthelemy R, Walch G. Fatty infiltration of the supraspinatus: a reliability study. J Shoulder Elbow Surg 2009;18:581-7.
- ↑ Kissenberth MJ, Rulewicz GJ, Hamilton SC, Bruch HE, Hawkins RJ. A positive tangent sign predicts the repairability of rotator cuff tears. J Shoulder Elbow Surg 2014;23:1023-7.
- ↑ Thomazeau H, Rolland Y, Lucas C, Duval JM, Langlais F. Atrophy of the supraspinatus belly. Assessment by MRI in 55 patients with rotator cuff pathology. Acta Orthop Scand 1996;67:264-8.
- ↑ Sheean AJ, Hartzler RU, Denard PJ, et al. Preoperative Radiographic Risk Factors for Incomplete Arthroscopic Supraspinatus Tendon Repair in Massive Rotator Cuff Tears. Arthroscopy 2018;34:1121-7.
- ↑ Melis B, DeFranco MJ, Chuinard C, Walch G. Natural history of fatty infiltration and atrophy of the supraspinatus muscle in rotator cuff tears. Clin Orthop Relat Res 2010;468:1498-505.
- ↑ Collin P, Treseder T, Lädermann A, et al. Neuropathy of the suprascapular nerve and massive rotator cuff tears: a prospective electromyographic study. J Shoulder Elbow Surg 2014;23:28-34.
- ↑ Lädermann A, Genevay M, Abrassart S, Schwitzguebel AJ. Supraspinatus Intramuscular Calcified Hematoma or Necrosis Associated with Tendon Tear. Case Rep Orthop 2015;2015:496313.
- ↑ Sheean AJ, Hartzler RU, Denard PJ, et al. Preoperative Radiographic Risk Factors for Incomplete Arthroscopic Supraspinatus Tendon Repair in Massive Rotator Cuff Tears. Arthroscopy 2018;34:1121-7.
- ↑ Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am 2005;87:1476-86.
- ↑ Tokish JM, Alexander TC, Kissenberth MJ, Hawkins RJ. Pseudoparalysis: a systematic review of term definitions, treatment approaches, and outcomes of management techniques. J Shoulder Elbow Surg 2017;26:e177-e87.
- ↑ Collin P, Matsumura N, Lädermann A, Denard PJ, Walch G. Relationship between massive chronic rotator cuff tear pattern and loss of active shoulder range of motion. J Shoulder Elbow Surg 2014;23:1195-202.
- ↑ Axe JM. Tendon transfers for irreparable rotator cuff tears: An update. EFORT Open Rev 2016;1:18-24.
- ↑ Levy O, Mullett H, Roberts S, Copeland S. The role of anterior deltoid reeducation in patients with massive irreparable degenerative rotator cuff tears. J Shoulder Elbow Surg 2008;17:863-70.
- ↑ Collin PG, Gain S, Nguyen Huu F, Lädermann A. Is rehabilitation effective in massive rotator cuff tears? Orthopaedics & traumatology, surgery & research : OTSR 2015;101:S203-5.
- ↑ Ainsworth R. Physiotherapy rehabilitation in patients with massive, irreparable rotator cuff tears. Musculoskeletal care 2006;4:140-51.
- ↑ Levy O, Mullett H, Roberts S, Copeland S. The role of anterior deltoid reeducation in patients with massive irreparable degenerative rotator cuff tears. J Shoulder Elbow Surg 2008;17:863-70.
- ↑ Yian EH, Sodl JF, Dionysian E, Schneeberger AG. Anterior deltoid reeducation for irreparable rotator cuff tears revisited. J Shoulder Elbow Surg 2017;26:1562-5.
- ↑ Collin PG, Gain S, Nguyen Huu F, Lädermann A. Is rehabilitation effective in massive rotator cuff tears? Orthopaedics & traumatology, surgery & research : OTSR 2015;101:S203-5.
- ↑ Boileau P, Maynou C, Balestro JC, et al. [Long head of the biceps pathology]. Rev Chir Orthop Reparatrice Appar Mot 2007;93:5S19-53.
- ↑ Walch G, Edwards TB, Boulahia A, Nove-Josserand L, Neyton L, Szabo I. Arthroscopic tenotomy of the long head of the biceps in the treatment of rotator cuff tears: clinical and radiographic results of 307 cases. J Shoulder Elbow Surg 2005;14:238-46.
- ↑ Boileau P, Baque F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am 2007;89:747-57.
- ↑ Scheibel M, Lichtenberg S, Habermeyer P. Reversed arthroscopic subacromial decompression for massive rotator cuff tears. J Shoulder Elbow Surg 2004;13:272-8.
- ↑ Berth A, Neumann W, Awiszus F, Pap G. Massive rotator cuff tears: functional outcome after debridement or arthroscopic partial repair. J Orthop Traumatol 2010;11:13-20.
- ↑ Chen KH, Chiang ER, Wang HY, Ma HL. Arthroscopic Partial Repair of Irreparable Rotator Cuff Tears: Factors Related to Greater Degree of Clinical Improvement at 2 Years of Follow-Up. Arthroscopy 2017;33:1949-55.
- ↑ Cuff DJ, Pupello DR, Santoni BG. Partial rotator cuff repair and biceps tenotomy for the treatment of patients with massive cuff tears and retained overhead elevation: midterm outcomes with a minimum 5 years of follow-up. J Shoulder Elbow Surg 2016;25:1803-9.
- ↑ Galasso O, Riccelli DA, De Gori M, et al. Quality of Life and Functional Results of Arthroscopic Partial Repair of Irreparable Rotator Cuff Tears. Arthroscopy 2017;33:261-8.
- ↑ Godeneche A, Freychet B, Lanzetti RM, Clechet J, Carrillon Y, Saffarini M. Should massive rotator cuff tears be reconstructed even when only partially repairable? Knee Surg Sports Traumatol Arthrosc 2017;25:2164-73.
- ↑ Henry P, Wasserstein D, Park S, et al. Arthroscopic Repair for Chronic Massive Rotator Cuff Tears: A Systematic Review. Arthroscopy 2015;31:2472-80.
- ↑ Shon MS, Koh KH, Lim TK, Kim WJ, Kim KC, Yoo JC. Arthroscopic Partial Repair of Irreparable Rotator Cuff Tears: Preoperative Factors Associated With Outcome Deterioration Over 2 Years. Am J Sports Med 2015;43:1965-75.
- ↑ Yoo JC, Ahn JH, Koh KH, Lim KS. Rotator cuff integrity after arthroscopic repair for large tears with less-than-optimal footprint coverage. Arthroscopy 2009;25:1093-100.