Shoulder:Glenohumeral Arthritis/Reverse Shoulder Arthroplasty
Contents
- 1 Bullet Points
- 2 Key words
- 3 History
- 4 Anecdotes
- 5 Human Development
- 6 Biomechanics
- 6.1 Arm Lengthening
- 6.2 Lateralization in Reverse Shoulder Arthroplasty
- 6.3 Neck-shaft angle
- 6.4 Range of motion after reverse shoulder arthroplasty: which combinations of humeral stem and glenosphere work the best?
- 7 Preoperative planning
- 8 Indications and Contraindications
- 8.1 Indications
- 8.1.1 Acute proximal humerus fracture
- 8.1.2 Malunited/nonunited proximal humerus fracture
- 8.1.3 Glenohumeral Osteoarthritis With Severe Glenoid Bone Loss
- 8.1.4 Chronic Locked Glenohumeral Joint Dislocation
- 8.1.5 Rheumatoid Arthritis With or Without Associated Rotator Cuff Tears
- 8.1.6 Revision Arthroplasty
- 8.1.7 Tumours
- 8.2 Contraindications
- 8.1 Indications
- 9 Approaches
- 10 Specific Conditions
- 11 Results
- 12 Complications
- 13 References
Bullet Points
- Indications for reverse shoulder arthroplasty expended. Weight-bearing patients, preoperative deltoid or acromial impairment, in certain circumstances, are not an absolute contraindication to reverse shoulder arthroplasty.
- Deltopectoral, anterosuperior (transdeltoid), and deltoid and subscapularis sparing approaches are currently used. Transacromial approach has been abandoned.
- Adequate deltoid tension obtained through restoration of humeral and arm length is one of the keys for postoperative function and prevention of instability following reverse shoulder arthroplasty. With a classic Grammont prosthesis postoperative humeral lengthening is approximately 2 mm and arm lengthening is approximately 24 mm.
- Subclinical neurologic lesions after Medial Glenoid/Medial Humerus Design are a frequent with consequence of lengthening with a drastically increasing prevalence above 40 mm of arm lengthening. Lateralized designs seem to be protective. Arm lengthening should be controlled with 0 to 2 cm being a reasonable goal to avoid postoperative neurological impairment.
- Medial Glenoid/Medial Humerus 155 degrees neck-shaft angle designs are progressively replaced by lateralized and lower neck-shaft angle (145-135 degrees) designs that theoretically attains, compared to traditional Grammont-type prosthesis, an optimal compromise in range of motion and soft tissue tension.
- Anterior forward flexion and Constant scores after reverse shoulder arthroplasty plateau at 6 months postoperative whereas internal and external rotation continue to improve up to 2 years postoperative. Several preoperative factors are correlated with postoperative range of motion.
- Previously, complications have been reported to affect 19% to 68% of patients and include acromial fracture, haematoma, infection, instability, mechanical baseplate failure, neurological injury, periprosthetic fracture and scapular notching. The rate of postoperative complications has dramatically decrease.
- The launch of a variety of reverse shoulder arthroplasty designs on the market has introduced a myriad of prosthetic configurations that has rendered analysis and delivery of universal guidelines difficult.
Key words
Reverse total shoulder arthroplasty; prosthesis; postoperative function; humerus and arm length; deltoid impairment; muscle insufficiency; complications; indications; indications, contraindications, impingement; humeral lateralization; glenoid; neck-shaft angle; function; range of motion; active forward flexion; predicting factors; results; clinical outcomes; weight-bearing joint; wheelchair; crutches.
History
Paul Grammont was born on April 1940 in Salins-les- Bains, in the northeastern part of France. He began medical studies in Lyon. Very quickly he became interested in surgery, and more specifically in orthopaedic surgery. He first became the fellow and then assistant of Professor Albert Trillat. He became a Professor of Orthopaedic Surgery and Traumatology in 1974 at the age of 34. He then moved to Dijon (France) where he became the Chairman of the Orthopaedic Department of the University Hospital. While he had few laboratory resources, he began many of his biomechanical shoulder experiments in his own garage. Grammont was creative: besides developing the reverse shoulder prosthesis,[1] he also developed an early patellofemoral prosthesis[2] and one of the first nails with a self-advancing mechanism designed to lengthen long bones like the tibia and the femur (Albizia nail).[3] Paul Grammont died in Lyon the 30 March 2013.
Anecdotes
This subsection does not exist. You can ask for it to be created, but consider checking the search results below to see whether the topic is already covered.
Human Development
During evolution the permanently orthograde posture has freed the human shoulder girdle of its quadruped functions. The anterior limbs became the upper limbs with the characteristics of a non-weight-bearing joint. Major bony and muscular adaptations occurred.[4] The scapulohumeral complex underwent drastic changes to facilitate prehension, leading to major bony and muscular modifications. A relative atrophy of the supraspinatus muscle occurred, as illustrated by a decrease in the scapular index.[5][6] The decrease in the effectiveness of the latter muscle was at the same time compensated for by the increase in size, mass, and lateral extension of the acromion process. The progressive distal migration of the point of insertion of the deltoid muscle and lateralization of the acromion indicates the more dominant position occupied by the deltoid with strengthening in particular of the middle deltoid abduction component.[7]
The glenohumeral joint is highly mobile and relatively unconstrained. Stability of the joint relies upon concavity-compression whereby the rotator cuff exerts a compressive force of the humeral head upon the glenoid. In the absence of concavity-compression, the unopposed contraction of the deltoid creates a force vector that displaces the head superiorly rather than in abduction. Depending on the type of rotator cuff lesion, a patient may present with pseudoparalysis.
To compensate the loss of function of the rotator cuff, several options have been proposed; the most reasonable, whenever possible, is to repair the rotator cuff. Good results are obtained in the vast majority of the cases with healing of the rotator cuff on the tuberosities. In some circumstances, rotator cuff repair is however contraindicated or technically impossible.
For instance, a rotator cuff insufficiency associated with pain and pseudoparalysis remains a challenging condition. It is extremely difficult, if not impossible, to obtain a functionally good result with a conventional prosthetic arthroplasty in this situation, where only a “limited goals surgery” is appropriate, a concept introduced by Neer.[8] Effectively, hemiarthroplasty provides satisfactory pain relief but poor motion,[9] whereas total anatomic shoulder arthroplasty is complicated with early loosening of the glenoid component.[10]
In order to provide active forward elevation above 90 degrees, the abduction role of the deltoid has to be increased. This can be obtained by several mechanisms, such as an osteotomy of the scapular spine[11] or more commonly by medializing the center of rotation the glenohumeral joint.[12] The concept of functional surgery is born from the latter option: whereas no effective anatomic solution exists, restoration of function has to be proposed through a novel morphology.
The first-generation of reverse shoulder arthroplasty have been implanted in Germany and France.[13][14] However, early loosening and mechanical complications forced to abandon their use. Nevertheless, successive improvements imagined by Grammont followed and, in 1991, a reverse shoulder arthroplasty called the “Delta III” has been developed.[15] The two major innovations were a large metal hemisphere with no neck on the glenoid side, and a small humeral polyethylene cup (covering less than half of the hemisphere), oriented with a nonanatomic inclination of 155 degree.
Biomechanics
Reverse shoulder arthroplasty, often used in multiply operated patients with distorted anatomy, imparts physiological and biomechanical changes that may increase the potential for complications.[16]
First, the arthroplasty position medializes and lowers the glenohumeral center of rotation, thereby increasing the lever of the deltoid muscle (Medial Glenoid/Medial Humerus Design). Deltoid tension, increased by the lowered center of rotation, increases muscle fiber recruitment of the anterior and posterior deltoid that compensates for a deficient rotator cuff (Figure). The medialization increased the deltoid moment arm up to 20%, and an inferior move increased the efficacy of the deltoid up to 30%.[17]

Second, to provide an inherently stable reverse shoulder arthroplasty, the weight bearing part is convex and the supported part concave (reversal of the ball and socket). The fixed nature of the glenosphere places torsional forces on the humerus that may affect humeral component instability.[19] The native spinning joint becomes a hinge joint, new configuration that leads to various impingements’ types and locations.
Third, the semi-constrained nature of the prosthesis restores glenohumeral stability which provides the stable fulcrum which is essential for active anterior elevation.
Finally, lengthening of the arm which provides space for range of motion of the proximal humerus,[20] enhances stability, and re-tensions the deltoid. The latter factor is critical due to the semi-constrained design of the prosthesis. The increase in compressive force between the humeral and glenoid components has a stabilizing effect.[21] Under such tension, the reverse glenoid component provides the stable fulcrum essential for shoulder anterior elevation and prosthesis stability.[22] This tension is determined by arm length.
Arm Lengthening
Failure to adequately tension the deltoid may result in prosthetic instability and poor function which are the most common clinically significant complications following reverse shoulder arthroplasty. On the other hand, other complications following reverse shoulder arthroplasty can be related to excessive deltoid tension such as neurological lesions, fractures of the acromion, or fixed abduction of the arm.[23][24] Adequate deltoid tension is thus accepted as a key to prosthetic function and stability.[25][26]
The glenosphere has to be implanted on the lower part of the glenoid to avoid notching and to improve rotation at 90 degrees of abduction.[27][28][29][30][31][32] The type of glenosphere (size, eccentricity) allowed the adjustment of arm length by several millimeters (about 1% of arm length). Consequently, the key factors for arm length are the height of the stem, type of stem, polyethylene thickness and the use of an augment or spacer. Collectively, these factors allow arm lengthening by up to several centimeters (about 10% of arm length).[33] The tension is thus determined by arm length. The latter is dependent of 1) the position of the glenosphere in the frontal plane (Figure), 2) the size of the glenosphere, 3) the use of an eccentric or inferiorly tilted glenosphere, 4) the use of a spacer, 5) the thickness of the polyethylene, 6) the height of humeral cut and stem implantation (Figure)[34] and prosthetic design (Figure).



From a clinical perspective lengthening of the arm and humerus, distalization angle, acromio-prosthesis distance (Figures) have been used as surrogates for deltoid tension since they intuitively correlate with deltoid tension and they have been correlated with functional outcome and risk of postoperative instability.[37][38][39] Most of these factors nowadays easily be to evaluated thank to navigation software.[40][41]


Lateralization in Reverse Shoulder Arthroplasty
The basic biomechanical principles of the Grammont reverse shoulder arthroplasty are a medialization of the glenohumeral center of rotation, the use of a larger ball on the glenoid component, and a lowering of the humerus. These principles increase the deltoid lever arm and provide a space for unrestricted range of motion of the proximal humerus and a stable fulcrum essential for active elevation and stability.[43] However, many complications, such as limited postoperative range of motion or impingement that could be attributed to the medialized glenoid design, have been reported in the literature.[44] To address these problems, several authors have proposed a change in the design of the Grammont prosthesis, promoting an increased bony or metallic glenoid offset.[45][46] Different methods to measure glenoid lateralization have been proposed.[47][48]
Definitions
It is important to understand the differences between humeral or glenoid lateralization. These factors can be used to predict range of motion and vary based on prosthesis and technical factors. Humeral lateralization is defined as the distance from the center of the polyethylene cup, and the lateral part of the greater tuberosity (Figure). It can be estimated by the lateralization shoulder angle (Figure).
It depends upon glenoid wearing, reaming and grafting, contact of the baseplate with the glenoid, the offset of the glenosphere and/or baseplate, the glenosphere diameter and tilt, the level of the humeral cut, the humeral neck-shaft angle, the humeral prosthetic design, the use of a spacer, the polyethylene thickness (humeral polyethylene socket offset), and the remaining proximal and lateral humeral bone stock.
At the other end of the spectrum, failure to adequately restore bone or prosthetic humeral lateralization may result in loss of humeral contour,[49] and deltoid shape curve and thus deltoid retensionning, and could lead to prosthetic instability and poor postoperative function.[50][51]
Neck-shaft angle
More anatomic neck-shaft angles decrease the rate of scapular notching and improve postoperative range of motion.[52][53]
The launch of this variety of designs on the market has introduced a myriad of prosthetic configurations that has rendered analysis and delivery of universal guidelines difficult.
Range of motion after reverse shoulder arthroplasty: which combinations of humeral stem and glenosphere work the best?
Introduction
The initial reverse shoulder arthroplasty design was excellent at restoring forward flexion but had several design-related complications including bony impingement and scapular notching,[55][56] instability,[57] acromial fractures,[58] limited range of motion (particularly internal and external rotation),[59][60] and humeral stem loosening.[61][56] Many of these have been attributed to the initial Grammont design which featured a medialized glenosphere and 155 degrees straight stem (Medial Glenoid/Medial Humerus Design).[62]
A variety of changes in prosthetic design have been proposed to address these issues either on the humeral side or on the glenoid side, the goal being to decrease scapular notching, maximize efficiency of the remaining rotator cuff, improve stability and improve range of motion. On the glenoid side authors have promoted increased lateralization either with bone or metal.[63][64] On the humeral side, a more anatomic humeral inclination (i.e. 145 or 135 degrees) and inlay and onlay systems designs has introduced a myriad of prosthetic configurations that has rendered analysis and delivery of universal guidelines difficult.
Therefore, the aim of this chapter is to evaluate the advantages and inconvenient of different reverse shoulder arthroplasty’s designs and to provide recommendations accordingly.
Glenoid Configuration
Glenoid configuration has an important effect on postoperative range of motion. The three most important variables are glenoid offset, eccentricity, and glenosphere size. None of these latter parameters influence significantly the measured bone strains at the glenoid near the bone-implant interface.[65]
Glenoid Offset (lateralization)
The initial Grammont-style reverse shoulder arthroplasty utilized a glenosphere with a medialized center of rotation. While this design reliably improved forward elevation, the high rate of scapular notching and internal and external rotation deficit observed with this design have been attributed to the medialized glenoid design.[44][66] To address these problems, glenoid lateralization have been proposed to decrease scapular notching, improve soft tissue tension, and increase impingement-free range of motion. The glenoid component is considered as lateralized if lateralization equals or exceed 5 mm compared to Grammont design.[67] It is important to note that this lateralization of the center of rotation is relative to the implant designed by Grammont, but still medialized compared to the native glenohumeral joint. Lateralization can be achieved with either the placement of bone medial to the baseplate (bone increase offset reverse shoulder arthroplasty (BIO-RSA))[68] or with metallic lateralization via the baseplate or glenosphere. While both have been associated with clinical improvement,[69][70] metallic lateralization appears may be less subject to displacement, particularly with lateralization beyond 5 mm.Denard PJ, Lederman E, Parsons BO et al. Finite element analysis of glenoid-sided lateralization in reverse shoulder arthroplasty. J Orthop Res 2017;35:1548-1555
Basic science studies show several benefits of lateralization. In both sawbone[71] and computer models,[64][72][73] lateralization improves range of motion in all directions.[53] Lateralization also leads to improvement in stability.[74]
The question remains how much lateralization is ideal. While clinical evidence is currently lacking, computer modeling suggests that 5 to 10 mm of lateralization relative to the native glenoid is ideal.[75][28][76] Nevertheless, clinical data to date has not necessarily proved that lateralization improves range of motion[18] or outcome scores[77] compared to a traditional reverse shoulder arthroplasty. Greiner et al. performed a randomized controlled trial of 17 Grammont reverse shoulder arthroplastys and 17 BIO-RSAs and reported no difference in Constant scores at 1 year postoperative.[78] In a retrospective study, Athwal et al. did not observe substantial range of motion, strength, or outcome scores.[79] The frequency of scapular notching, however, was significantly higher (P=.022) in the reverse shoulder arthroplasty cohort than in the BIO-RSA cohort: 75% versus 40%.[80] This finding has been also reported by Zitkovsky et al.[81] At 10 years follow-up, Kennon et al. confirmed that scapular notching rates are significantly higher with medialized components compare to lateralized ones (77% in vs. 47%, P= .013).[82] Notably, all of the these studies utilized a 155 degrees humeral prothesis and thus further comparative studies are required with 135 and/or 145 degrees protheses.
Glenosphere Eccentricity
Inferior eccentric positioning of the glenosphere can also be used to decrease the adduction deficit and thus reduce scapular notching.[23, 27] Mizuno et al. previously reported that an inferiorly eccentric glenosphere reduced the severity of scapular notching with a 155 degrees prosthesis,[39] improving thus postoperative rotations elbow at side.[31] While the differences are small, the eccentric glenosphere provided the greatest ability to limit scapular notching while maximizing range of motion by increasing the subacromial space.[27, 33] Abduction is effectively positively correlated with acromiohumeral distance (r = 0.93; p < 0.001) which is increased with an eccentric glenosphere.[28] Rotation in abduction is important to activities of daily living. Interestingly, the latter are impossible in most configurations due to inexistent subacromial space (Figure). Postero-inferior eccentricity can improve also extension and could favorize as well internal rotation hand in the back (Figure).
Figure: Rotations rotation at 90 degrees of abduction might be impossible due to inexistent subacromial space. Eccentric positioning of the glenosphere creates subacromial space.
Figure: Postero-inferior eccentricity improves extension. A) Inferior eccentricity alone (yellow arrow) allows 52 degrees of extension. B) 40 degrees of postero-inferior eccentricity (yellow arrow) improves extension to 73 degrees.
It is important to note, however, that inferior overhang of the glenosphere can be achieved either by an eccentric glenosphere or by baseplate position. Conversely, an eccentric glenosphere with an improperly positioned superior baseplate will not provide clinical benefit. Thus, the surgeon must be cognizant of both the overhang of the given glenosphere relative the selected baseplate, as well as any eccentricity in the glenosphere. Furthermore, the benefits of overhang or eccentricity must be weighed against the risks of nerve injury and acromial fracture associated with arm lengthening. The ideal amount of overhang relative to the glenoid appears to be about 2.5 mm based on clinical evidence.[20]
Glenosphere size
The size of the glenosphere influences theoretically and clinically postoperative range of motion. Lädermann et al. found that a small glenosphere (36 mm) improves external rotation in abduction.[27] However, with the elbow at side, larger diameter glenospheres have been shown to provide a greater impingement-free arc of motion, and decrease scapular notching in biomechanical studies. Werner et al. reported superior values for extension and external rotation with a 39 mm glenosphere compare to a 36 mm glenosphere.[48] mm). Berhouet et al. demonstrated in a cadaveric study that a 42 mm glenosphere was associated with improved rotational range of motion compared to a 36 mm glenosphere (p <0.05).[3] Another study comparing functional scores and range of motion differences between two groups of patients, one receiving a 36 mm glenosphere and the other receiving a 44 mm glenosphere, found that patients with the larger glenosphere had a 12 degrees increase in external rotation in adduction compared to those with the smaller glenosphere (p <.001).[41] Similarly, Mollon et al. showed that a 42 mm glenosphere size generated a 15 degree improvement in forward flexion and a 6 degree improvement in external rotation compared to the 38 mm size, with an overall improvement in pain scores.[40] Finally, a study by Haidamous et al. demonstrated that larger glenosphere size and inferior positioning as well as posterior humeral offset are associated with improved postoperative range of motion following reverse shoulder arthroplasty with a 135 degrees humeral component.[20] Nevertheless, larger glenospheres limit abduction and rotations in abduction and are prone to higher volumetric wear rates and experienced greater polyethylene volume loss.[18] Additionally, one must consider patient size. Overstuffing can occur. Matsuki et al., for instance, demonstrated that small- and large-stature patients achieved lower improvement in range of motion with an RSA system with only 2 glenosphere sizes (38 and 42) likely because the small patients were overstuffed and the large patients did not have large enough glenospheres and/or lateralization.[37]
Humeral Stem Designs
The primary humeral stem variables include stem geometry, neck-shaft angle, inlay versus onlay configuration, and humeral spacers.
Stem geometry
Short curved stems were initially developed to facilitate implantation, maintain bone stock, and preserve rotator cuff insertion.[25] These stems also change humeral offset based on their positioning in the humeral canal. In one study an onlay curve stem lead to a 7-mm increase in humeral offset compared with a traditional inlay straight Grammont prosthesis.[26] Curve stems decrease the acromiohumeral distance, which may lead to acromial impingement at small abduction angles.[26] On the other hand, humeral lateralization can be useful to compensate for medialization in case of bone loss (Figure) and has been theorized to improve the mechanics of the remaining rotator cuff and deltoid musculature.[45] Stem design appeared to have also a substantial effect on abduction, as combinations with the straight Grammont stem had greater abduction (73–80%), compared to those with the onlay curved stem (54–69%).[28]
Figure 3: Humeral lateralization can help to restore global lateralization in case of glenoid bone loss. A) Massive glenoid bone loss compensated by glenoid allograft and a straight stem (Lateral Glenoid/Medial Humerus concept). Observe that the central peg hardly reaches the native glenoid. Such construct has a potential for failure (B). Another option would have been to use a smaller glenoid graft and a curved stem (Lateral Glenoid/Lateral Humerus).
Neck-shaft angle (inclination)
The Grammont reverse shoulder arthroplasty was designed as a non-anatomic implant with a relative valgus humeral neck inclination of 155 degrees. Based on the work by Gutierrez et al.,[17] neck-shaft angle has decreased in modern prosthetic designs to a more varus or anatomic inclination of 145 or 135 degrees).
The neck shaft angle is a major factor influencing length of the arm,[30] but has little effect on humeral lateralization; by changing inclination from 155 degrees to 135 degrees within a onlay design, humeral offset only increased by about 2 mm.[26]
Theoretically, compared to low neck shaft angle stems, higher inclinations (155 degrees) increased abduction by 100% and external rotation in abduction, regardless of glenosphere designs.[26, 33] This finding is important as such external rotation is a major factor in the ability to perform activities of daily activities such as hair care and facial grooming. However, a 155 degrees is associated with decreased adduction external rotation at the side and[26, 42, 49] extension due to medial bony impingement (which also leads to scapular notching).[15, 28, 33, 44, 47, 49] Lateralization obtained via a lower neck shaft angle increases adduction, by 357% between a 155 degrees prosthesis compared with a 135 degrees prosthesis. Also, an increase in extension, of 381%, and external rotation elbow at side, of 116%, are observed with a la 135 degrees prosthesis.[26] Such finding are important as external rotation with the elbow at the side and extension led to friction between the scapular pillar and the polyethylene insert. Even if this friction phenomenon does not limit range of motion, it likely contributes to progressive polyethylene wear and scapular notching.[31] Reducing the neck-shaft angle can however have some negative effects on reverse shoulder arthroplasty contact mechanics. The contact area is reduced by 29% for 155 degrees to 145 degrees and by 59% for 155 degrees to 135 degrees. Consequently, there is an increased maximum contact stress by 71% for 155 degrees to 145 degrees and by 286% for 155 to 135 degrees.[34]
Gobezie et al. published the results of a randomized controlled trial comparing humeral inclination of 135 degrees to 155 degrees among patients undergoing reverse shoulder arthroplasty with a neutral glenosphere (no lateralization) and found no significant difference in forward flexion, external rotation, or functional outcomes.[13] They and other studies have confirmed that scapular notching is decreased with a 135 degrees prothesis. [9, 13, 51] A systematic review of 2222 shoulders comparing 135 degrees and 155 degrees prostheses reported higher rates of scapular notching in the 155 degrees group (16.8% vs. 2.8%), improved external rotation in the 135 degrees group, and no difference in instability of forward flexion between groups.[9]
Lastly, in case of fracture, reverse shoulder arthroplasty with a 135 degrees neck shaft angle is associated with higher tuberosity healing rates compared to 145 degrees or 155 degrees.[43]
Onlay vs Inlay
Compared to on inlay design, an onlay humeral design with the same 155 degrees inclination increased humeral offset by 6.6 mm.[26] Acromiohumeral distance varied by 9.8 mm with the smallest occurring with the onlay 135 degrees model and the largest occurring with a Grammont inlay 155 degrees. Compared to the inlay design, an onlay humeral design with the same 155 degrees inclination decreased the acromioclavicular distance by 4.1 mm. Compared to the onlay 155 degrees model, with the inlay 155 degrees model there was a 10 degree decrease (77.8 to 67.9 degrees) in abduction and a 5 degree (range, −15.3 to −20.2 degrees) increase in adduction.[26]
Clinically, Beltrame et al. conducted a prospective clinical study comparing onlay and inlay steams. They found that onlay design 145 degrees may provide better active external rotation, extension, adduction.[2] However, there are numerous bias in their study (i.e. different neck shaft angle and stem lateralization) that prevent integration of their results in the present analysis.
A retrospective review reported a trend towards a higher rate of acromial fractures among patients with an onlay (12%) as opposed to inlay (4%) system.[36] In a retrospective comparative radiological study, Haidamous et al. showed similarly that an onlay humeral stem design resulted in a 10 mm increase in distalization compared to an inlay humeral stem, and a 2.5 times (11.9% vs 4.7%) increased risk of scapular spine fracture.[19] It seems thus that the combination of lateralization and distalization in an onlay system dramatically increases the incidence of scapular spine fractures.
Lengthening of the supraspinatus and infraspinatus is systematically observed with an onlay design. It is greatest using onlay stems (7–30%) and lateralized glenospheres (13–31%).[28] Subscapularis lengthening is observed for onlay stems combined with lateralized glenospheres (5–9%), while excessive subscapularis shortening is observed for the inlay stem combined with all glenospheres except the lateralized design (> 15%).[28]
Polyethylene Insert
Since inferior impingement between the polyethylene and the scapula is systematic with the arm at the side, another potential way to limit friction and notching in external rotation is to create a notch in the polyethylene inferiorly between 3 and 9 o’clock as has been done in some prostheses (e.g., Arrow and SMR). Another solution to increase range of motion is to reduce the depth of the polyethylene inlay. De Wilde et al. found that for every 3-mm decrease in depth of polyethylene cup, ROM increased by 12 degrees.[83]
Discussion
The literature is controversial with regard to possibilities of regaining range of motion following reverse shoulder arthroplasty. While prosthetic designs are varied and lead to substantial changes in computer models, many of the theoretical advantages have not been confirmed clinically. Table summarizes implant design considerations to improve range of motion. The optimal compromise in range of motion for a primary reverse shoulder arthroplasty without bone loss could be a Lateral Glenoid/Medial (or Intermediate) Humerus design with a low neck shaft angle (145-135 degrees) and an inlay concept. However, all prosthetic designs should be considered on a case-by-case basis to optimize outcome. Glenoid and humeral prosthetic design has to be chosen depending of pre- and intra-operative factors including patient expectations, bone morphology, soft tissue state such as rotator cuff or nerve, approaches, surgical exposure, etc. It may for example not be possible to utilize a large glenosphere in all patients as they may not be appropriate for the anatomy of smaller individuals and might be more technically challenging. As a result, the surgeon must continuously weigh the benefits and possibilities of available implant-related variables regarding patient’s specific conditions. The systematic use of patient-specific patients specific instrumentation and navigation as well as preoperative determination of components are obviously the next steps in providing more accurate component positioning and size and thus improving range of motion. Despite the advances made by glenoid lateralization and inferiorization, there remains ample opportunity for continued improvement and innovation in prosthetic design.
Preoperative planning
Preoperative planning is mandatory as it allowed to improve range of motion.[84] To guarantee the best possible functional results, restoration of the appropriate humeral and arm length, a and free range of motion should be the goal.[85][86][87] Even if the available software do not take into account soft tissue (stiffness, fatty infiltration,…), they are already able plan analyze lateralization and distalization.[88][89]
Indications and Contraindications
Indications
The reverse shoulder arthroplasty is a powerful tool that has opened new barriers, especially for reconstructive shoulder surgery. Traditionally, the ideal candidate is a patient above 70 years old with symptomatic cuff tear arthropathy. Appropriate candidates now include young patients, who have shown excellent clinical improvement with high implant survivorship of up to 10 years.[90][91][92][93][94][95]
Many pathologies that could not be treated previously found a solution through this design, and indications are currently expanding. It is now used for various conditions such as failed total shoulder arthroplasty or hemiarthroplasty, complex proximal humeral fractures and defective fracture union or nonunion, chronic locked dislocation, immunological arthritis with or without associated rotator cuff tears, failed or irreparable massive rotator cuff tears, and tumors.[96]
Acute proximal humerus fracture
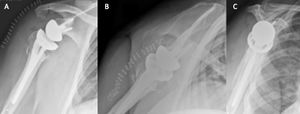
Reverse shoulder arthroplasty is a more reliable treatment than hemiarthroplasty for complex proximal humerus fractures at least in elderly patients because its functional outcomes appear to depend less on tuberosity healing and rotator cuff integrity (Figure).[97]
Malunited/nonunited proximal humerus fracture
Surgical options to address malunited proximal humerus fractures are determined largely by the existing deformity. They can be categorized broadly as humeral head-preserving techniques (e.g. osteotomies, soft-tissue releases and removal of bony protuberances) or humeral head-sacrificing techniques. Amongst the latter, reverse shoulder arthroplasty proved to be the most reliable.
Glenohumeral Osteoarthritis With Severe Glenoid Bone Loss
The use of reverse shoulder arthroplasty in patients with severe glenoid bone loss and osteoarthritis is the best option. Excellent results have been reported in patients with osteoarthritis, an intact rotator cuff and substantial glenoid bone loss treated with reverse shoulder arthroplasty with or without bone grafting (Video).

Chronic Locked Glenohumeral Joint Dislocation
Chronic locked glenohumeral dislocation can also be treated with reverse shoulder arthroplasty (Figure).[98]
Rheumatoid Arthritis With or Without Associated Rotator Cuff Tears
Excellent to satisfactory results have been reported for patients with rheumatoid arthritis treating with reverse shoulder arthroplasty. There is no higher complication rates as compared to reverse shoulder arthroplasty in cuff tear arthropathy.[99]
Revision Arthroplasty
Revision surgery after primary shoulder arthroplasty (i.e. hemiarthroplasty, resurfacing or total shoulder arthroplasty) produced high patient satisfaction (Figure). It is however associated with higher complication and failure rates compared with reverse shoulder arthroplasty for patients without previous arthroplasty.[100]
Tumours
Reverse shoulder arthroplasty is an acceptable option for patients after wide resection of the proximal humerus and rotator cuff tendons for malignant bone tumours. However, a prerequisite for the ability to implant a reverse shoulder arthroplasty in these cases requires preservation of the axillary nerve and deltoid muscle to be successful.[101][102]
Contraindications
Absolute contraindications include general factors such as non compliance patients (severe psychiatric/neurologic disability, substance abuse), neuro-arthropathies (Charcot) and high patient morbidity (ASA 4+), and local factors like an uncontrolled active infection and substantial deltoid insufficiency because of the very high probability of recurrent instability and the minimal potential gain in function.[103]
Approaches
Introduction
Reverse shoulder arthroplasty can be performed through several approaches, the deltopectoral and anterosuperior being the most common, each with their advantages and disadvantages. The preparation is standardized for all approaches. The patient lies in the beach chair position with a 60° tilt of the chest, at the lateral extremity of the table, leaving the anterior and posterior sides of the shoulder free from obstruction. The elbow must be free from any support to enable the operating assistant to apply a proximally directed force at the elbow allowing proximal subluxation of the humeral head. The front arm rests on an armrest and is draped free.
Deltopectoral Approach
The deltopectoral approach allows for increased visibility and accessibility of the humerus, better positioning of the glenoid component, reduced implant loosening and scapular notching, and does not compromise the deltoid, which is the important motor of the shoulder. This approach tenotomises the subscapularis or osteotomizes the lesser tuberosity. Failure (observed in 45% of cases, Collin, unpublished data) and dysfunction of the repaired subscapularis remains a concern after both tenotomy and lesser tuberosity osteotomy despite multiple variations in subscapularis takedown and reattachment techniques. Neurologic atrophy and fatty infiltration of the subscapularis muscle belly have been also reported to causes pain and impaired function.
Surgical technique
The deltopectoral approach consisted of a 10 to 15 cm skin incision being made from the coracoid process toward the deltoid insertion. The infraclavicular fossa (Mohrenheim fossa) is found, the cephalic vein identified and the consistent medial branches, which give the appearance of the Mercedes Benz symbol, are ligated. A self-retaining retractor is used to maintain exposure between the deltoid and pectoralis major. The subacromial bursa was resected to allow placement of a Hohmann retractor under the deltoid over the top of the coracoid process. The arm was abducted and internally rotated. The subacromial bursa is resected to allow placement of a Brown-Deltoid retractor.
Subscapularis Tenotomy
This subsection does not exist. You can ask for it to be created, but consider checking the search results below to see whether the topic is already covered.
Osteotomy of the Lesser Tuberosity
The osteotomy is initiated at the bicipital groove with a 2-mm saw blade and then completed with a curved osteotome. An approximately 2.5 cm2 in the coronal plane and 5 mm thick fleck of lesser tuberosity is taken such that the osteotomy entered the joint medially without violating the humeral head.[104][105]
A complete release of the subscapularis tendon is then performed and the tendon is pushed in the subscapularis fossa. A glenoid retractor is placed anteriorly. The humeral head is resected with a guide or a free-handed anatomic cut respecting native humeral head version and inclination.
Subscapularis Repair
This subsection does not exist. You can ask for it to be created, but consider checking the search results below to see whether the topic is already covered. Faire une video
Lesser Osteotomy Repair
Before placement of the humeral stem, two holes are created with a 2-mm drill bit in the bicipital groove at the superior and inferior aspects of the lesser tuberosity osteotomy. One hole was created in the metaphysis just medial to the lesser tuberosity osteotomy. The sutures are then passed from lateral to medial by entering the bicipital groove, passing around the humeral stem, and exiting medially (Figure). A racking hitch is positioned to rest in the bicipital groove. The two sutures are passed through the subscapularis just medial to the lesser tuberosity osteotomy. The needle is removed from each construct to leave two superior and two inferior limbs (Figure). Then, one of the superior limbs and one of the inferior limbs were shuttled through the superior racking hitch knot (Figure). The suture limbs are passed through a tensioner to remove slack and to tension the repair (Figure).
Anterosuperior (Transdeltoid) Approach
Molé et al. reported superolateral approach that has the main advantage of better post-operative stability, because the anterior structures, including ligament complexes, are preserved.[106] This approach is different from the transacromial approach originally described by Grammont and Baulot[107] and the anterosuperior approach described by Mackenzie.[108] While this technique has shown good results, it involves splitting of the deltoid muscle with the risk of weakening of the anterior deltoid (mechanical or neurologic by damage to the distal branches of axillary nerve) and improper postoperative function.
Surgical technique
The skin incision extends from the posterior part of the acromioclavicular joint. It is 9 cm long and runs along the axis of the arm. The surgeon dissociates the anterior deltoid fibers and positions a stop suture on the distal portion of the dissociation to prevent any injury of the axillary nerve. The deltoid muscle fibers are divided to open and excise the subacromial bursa and the anterior deltoid from the anterior edge of the acromion and takes away the proximal attachment of the coracoacromial ligament as one piece. The humeral head osteotomy should be generous to allow optimal exposure of the glenoid. Glenoid exposure is completed, labrum is resected, and peripheral capsular release performed. The inferior labrum is carefully released with a knife while maintaining contact with the bony rim and avoiding electric cautery, considering the proximity of the axillary nerve, which is not visualized. This allows the positioning of a hooked retractor that presses the humeral epiphysis, which is protected by a trial humeral prosthesis. Once the glenoid implant is in place, the surgeon subluxates the humerus superiorly and anteriorly and cuffs the trial humeral stem with a trial insert; the reduction enables the surgeon to test the stability and tension. At the end of surgery, the deltoid is closed using laterolateral sutures.
Deltoid and Subscapularis Sparing Approach (Subscapularis On)
Indications for subscapularis-on approach were all types of primary reverse shoulder with an intact subscapularis.
Surgical technique The skin incision extends from the tip of the coracoid process and runs along the axis of the arm. A deltopectoral approach is performed (please refer to deltopectoral approach). After excision of the bursa, the surgeon explores the cuff through rotator interval. Once an intact subscapularis is confirmed, deep dissection is carried out either through the supraspinatus tear or after detaching it. With the arm held in extension and adduction, two long blunt-tipped Hohmann retractors are placed around the humeral head, allowing clear exposure of the proximal humerus (Figure).
The humerus is prepared to accommodate a stem. After a retroversion guide placement, the level of the humeral head osteotomy is marked with an electrocautery device (Figure), and a free-hand osteotomy is performed. The humeral head osteotomy should be generous to allow optimal exposure of the glenoid.
The humeral shaft is then prepared (Figure).
File:Sensitive-content.pngal, preparation of the humeral shaft is completed using only compactors.
If the initial osteotomy is too shallow or the inclination is suboptimal, it is then revised to maximize the anatomic fit between the prosthesis and the bone. After preparing the humerus, a trial humeral prosthesis is inserted in humeral canal to protect the humeral epiphysis during glenoid preparation. Cartilage removal, labrum resection, and peripheral capsular release are then completed sequentially. Tight inferior glenohumeral ligaments, which may prevent adequate exposure of the glenoid or post-operative shoulder mobility, are released using an electrocautery device with close contact with the inferior glenoid rim. A forked retractor is then inserted inferiorly to maintain visualization and accessibility to the glenoid (Figure).
This maneuver pushes the humeral epiphysis inferiorly or antero-inferiorly (compared to standard deltopectoral approach in which the humeral head is dislocated posteriorly) for better visualization of the glenoid. The glenoid is prepared according to the recommended surgical technique to obtain neutral inclination and version. Preoperative planning software is used to determine the amount of inferior tilt and whether an augmented baseplate is required. The baseplate is secured onto the glenoid with non-locking and locking peripheral screws. An eccentric 36 or 39 mm glenosphere is used to limit impingement in adduction, extension and external rotation. It is not recommended implanting a larger glenosphere as the excessive lateralization may hinder access to the humerus. The glenosphere is impacted into the baseplate. Once the glenoid implant is in place, the surgeon subluxs the humerus superiorly and anteriorly. A stem is inserted. The shoulder is reduced via gentle traction on the arm and range of motion tested in all planes to ensure stability and confirm the prosthesis moves easily without impingement. The prothesis is then dislocated for final implantation of a definitive polyethylene. Osteophytes are removed and lateral tuberoplasty can be performed to maximize flexibility and avoid bony impingement. The surgical incision measures about 7 to 10 cm (Figure).
Postoperative Rehabilitation
By using this subscapularis-on approach, patients do not require any immobilization with a sling following the operation. Immediate active motion in all planes is allowed post-operatively.
Complications
Tuberosity avulsions that require suture cerclage can been observed (Figure).
Advantages of Subscapularis-on Approach
There are several reasons why the integrity of the subscapularis tendon should be maintained when performing a reverse shoulder arthroplasty. First, acute muscle lengthening related to the non-anatomic design of the prosthesis plays a role. The muscle lengthening occurs mainly in the supraspinatus (19 mm with a bony increased offset reverse shoulder arthroplasty (BIO-RSA) implant), followed by the upper part of the subscapularis, which accounts for 70% or more of the strength and function of the subscapular muscle-tendon unit. Muscle lengthening could theoretically make reinsertion of the subscapularis more challenging, particularly with lateral offset designs. Secondly, the inferior part of the subscapularis has no tendon macroscopically; the muscle attaches directly to the bone, making reinsertion difficult. Healing rate is consequently low, at around 55%. Thirdly, the subscapularis is described as being the largest muscle in the rotator cuff and stronger (53% of global strength of the rotator cuff) than the supraspinatus, infraspinatus and teres minor combined.[109] If a muscle has to be divided, it seems logical to sacrifice the supraspinatus that accounts for only 14% of the global strength.[110] Fourth, the subscapularis plays a crucial role in anterior elevation. Collin et al. previously demonstrated that the subscapularis is the most important rotator cuff muscle for elevation in native shoulders.[111]
Disadvantages of Subscapularis-on Approach
The main disadvantage of the subscapularis-on technique is limited surgical exposure. Even though specialized jigs were not required for the above-mentioned technique, the development of specifically-designed instrumentation for this procedure seems necessary. Moreover, limited exposure prevents the use of patient-specific surgical guides. Development of less invasive guides or navigation systems may become inevitable in the future. Even if good exposure of the humeral head is achieved, the free-hand humeral osteotomy can be problematic. Subscapularis-on approach is technically challenging in certain cases (e.g. stiff shoulders, small patients) and may not be practical or possible in all circumstances. Intra-operatively, important lateralization (> 5 mm) of the glenoid is impossible, as subsequent exposure of the humerus is insufficient to implant the stem.
Specific Conditions
Reverse Shoulder Arthroplasty in Patients with Preoperative Deltoid Impairment
Definition, Causes, and Classification of Deltoid Impairment
The deltoid is critical for shoulder motion and any pathology involving this muscle is highly detrimental to normal glenohumeral function. It generates over 50% of the force necessary to elevate the arm in scapula plane in a normal shoulder and is the only muscle remaining to provide an abduction moment in patients with massive rotator cuff tears.[112] Deltoid impairment is defined as any condition which compromise its physiological function. Such impairment may be permanent or transient and can occur from a variety of conditions.
The deltoid muscle may be shortened upon itself and lose function by disruption of normal length-tension relationships (Figure).
Effectively, as the Blix curve describes, maintenance of length is required for a muscle to generate adequate tension.[113] Therefore shortening either by proximal migration of the deltoid insertion (rotator cuff arthropathy) or distal migration of the origin (scapular spine fracture) will compromise deltoid function. Proximal migration in particular can be considered a transient cause of deltoid impairment since it can be treated with reverse shoulder arthroplasty. Distal migration, on the other hand, may be permanent or transient depending on the situation.
In the most severe conditions, part or all of the deltoid muscle may be completely absent. Such permanent impairment is rare but may be observed following deltoid muscular flap transfer (for irreparable rotator cuff tears, Figure)[114][115][116] or following tumor resection (Figure).


One of the most common forms of deltoid impairment seen clinically is disruption of the muscle origin (without removal of the entire muscle belly). This most commonly occurs in the postsurgical setting after an open rotator cuff repair in which a deltoid split approach is used and part of the deltoid origin is take-down to gain exposure (Figure).[117]

Failure of the deltoid to heal back to the acromion can easily be appreciated clinically by a defect to palpation. Additionally, deltoid insertion disruption can occur through chronic attritional rupture as in chronic rotator cuff arthropathy with anterosuperior escape,[118][119] or following trauma (Figure).[120][121]
The deltoid muscle may be globally impaired in the setting of persistent denervation,[122] grade 3 or 4 fatty infiltration,[123] previous surgical approach, trauma (Figure), post radiation syndrome, or myopathy (myositis, Parkinson, Duchenne muscular dystrophy, etc.).[124]
Once the etiology is determined, the deltoid impairment should be then classified according to its location and extent. Lädermann et al.[125] proposed a classification for deltoid impairment based on location: type 1 corresponds to an impairment localized anteriorly, type 2 an anterior and middle one, type 3 involves only the middle deltoid, and type 4 is a global impairment (Figure). As discussed subsequently, this classification related to prognosis with type 4 in particular having a poorer function following reverse shoulder arthroplasty.
Results
Glanzmann et al. first published a case report of the results of a reverse shoulder arthroplasty after deltoid muscle flap transfer.[126] At two years follow-up, the patient was satisfied and had a Constant score of 62 points, suggesting that the entire deltoid may not be necessary for a successful outcome. Tay and Collin also described successful results of a reverse shoulder arthroplasty implanted in the setting of an irreparable rupture of the middle portion of the deltoid muscle.[127] No intra- or postoperative complication was noticed. At two years follow-up, the patient was pain free, had active anterior elevation of 150 degrees, and the Constant score was 65 points. Gulotta et al. reported in their biomechanical study that scapular plane elevation may still be possible following a reverse shoulder arthroplasty in the setting of anterior deltoid deficiency. When the anterior deltoid is deficient, there is a compensatory increase in the force required by the subscapularis and middle deltoid.[128] In this condition, surgeons should focus on preserving the subscapularis as much as possible during approach of reverse shoulder arthroplasty. Whatley et al. reported three cases who had postoperative rupture of the anterolateral deltoid following failed mini-open or open rotator cuff repairs. Successful repair of the deltoid was achieved using a transosseous suture repair in all three patients.[129] Essilfie et al. presented a case with deltoid failure after anatomical total shoulder arthroplasty revised with reverse shoulder arthroplasty. His ASES score after reverse shoulder arthroplasty was better than historical outcomes for resection arthroplasty and glenohumeral arthrodesis.[130] Lattisimus dorsi muscle transfer can also provide an augmentation in patients with deltoid insufficiency.[131][132] Dosari et al. presented a patient with a history of gunshot injury and loss of most of his shoulder bony and muscular structures. Due to deltoid muscle deficiency, the patient underwent lattisimus dorsi muscle flap followed by reverse shoulder arthroplasty with successful result.[133] Deltoid reconstruction at the same time of reverse shoulder arthroplasty is also a viable choice as a salvage procedure for patients with deltoid deficiency.[134] Marinello suggested if less than 50% of any part of the anterior or middle deltoid was involved (≤3 cm), reattachment or reconstruction was not needed. If all of the anterior and/or middle deltoid were involved, then reattachment or reconstruction was indicated.[135] In a multicentered study, Lädermann et al. reviewed 49 patients (49 shoulders) at a mean of 38 ± 30 months postoperative following reverse shoulder arthroplasty in the setting of deltoid impairment.[136] Postoperative complications occurred in nine (18%) patients, including two postoperative dislocations and two acute postoperative neurological lesions. Five (10%) patients required additional surgery. Active forward elevation and Constant score improve significantly. However, these values are significantly lower for patients suffering from global deltoid impairment (type 4) compared to types 1 through 3. The mean postoperative forward elevation was lower in the setting of global deltoid impairment (70 degrees) compared to partial impairment (127 degrees, 136 degrees and 125 degrees, groups 1-3 respectively) (P=.002). The postoperative Constant score was lower in the setting of global impairment (41) compared to partial impairment (57, 63 and 68, groups 1-3 respectively) (P=.006). Overall, the rate of patient satisfaction was 98% at final follow-up.[137] Schneeberger et al. retrospectively reviewed the outcome of 19 patients treated with reverse shoulder arthroplasty after failed deltoid flap reconstruction.[138] They noticed a high rate of complication (37%), including one instability. Nonetheless, at a mean follow-up of 4.5 years, only two patients had moderate to severe pain, all patients regained anterior active elevation above 90 degrees, and 15 of 19 patients were very satisfied.
It seems that the most important factor for postoperative result is the extent of the lesion, and not its cause. Interestingly, patient satisfaction is high in all publications on reverse shoulder arthroplasty in the setting of deltoid impairment. However, this is likely related to very poor preoperative function and moderate preoperative expectations of this population.
Acromial Insufficiency
Pre- or postoperative acromial pathology, which could theoretically compromise deltoid condition and affect the proper function of the prosthesis, is of legitimate concern.
Preoperative
Postoperative fractures occur at least in 3% of cases and their causes are numerous. Preoperatively, the acromion may be subject to a congenital or acquired abnormality such as an os acromiale. It can also already be eroded, fragmented or even fractured from the underlying head in case of cuff tear arthropathy (Figure), or osteoporosis-induced insufficiency.
Postoperative
It has been suggested that these fractures may be the result of a stress coming from the tip of the superior metaglene fixation screw.[139] Another risk factor is osteoporosis[140][141] The role of prosthetic design, distalization, global lateralization and Glenoid medicalization is still debated.[142][143]
Acromial fractures can be classified as avulsion fractures of the anterior acromion (Type I), fractures of the acromion posterior to the acromioclavicular joint (Type II) and fractures of the scapular spine (Type III).[144]
The symptomatology usually appears within the first year with sudden pain and decrease of function. The localization of the former is typically posterior. The fracture is best seen on an axillary lateral view to differentiate acromial fracture from scapular spine fracture (Figure).
The use of positron emission tomography-computed tomography is helpful in diagnosis of non-displaced fractures.
Good results have surprisingly been reported in patients with preoperative acquired or congenital acromial pathology or postoperative acromial fracture.[145] This can be explained by the persistent attachment of the deltoid to the spine of the scapula and clavicle and the more predominant postoperative scapulothoracic motion compared to the glenohumeral one. In case of postoperative acromial or scapular fractures, results are usually disappointing.[146]
The best treatment option for acromial fractures is thus conservative, as it does not lead to major shoulder dysfunction. Outcome of scapular spine fractures are more unpredictable with displacement of the bony support for the entire deltoid, pain and dysfunction. Consequently, some authors recommend open reduction, internal fixation and allograft associated with postoperative immobilization on a 60° abduction splint in order to avoid nonunion and acromiohumeral contact secondary to inferior acromial tilt.[147]
Latissimus Dorsi Transfert in Combination with the Reverse Shoulder Arthroplasty
This subsection does not exist. You can ask for it to be created, but consider checking the search results below to see whether the topic is already covered.
Reverse Shoulder Arthroplasty in Weight Bearing Patients
This subsection does not exist. You can ask for it to be created, but consider checking the search results below to see whether the topic is already covered.
Results
With a mean anterior forward flexion of 137 degrees and a mean external rotation elbow at the side of 6 degrees, reverse shoulder arthroplasty typically provides satisfactory clinical outcomes for a variety of complex shoulder diagnoses associated with severe pain and limitation of range of motion.[148] However, some patients have had unexpectedly poor functional improvements after reverse shoulder arthroplasty.[149] Poor postoperative range of motion following reverse shoulder arthroplasty, has been associated with younger age,[150] gender,[151] surgeon experience,[152] preoperative diagnosis such as posttraumatic arthritis and revision arthroplasty,[153][154] pre- and intraoperative range of motion or deltoid impairment,[155][156] postoperative arm lengthening[157][158][159] or neurological lesion.[160][161] Surgery of the non-dominant side, lower preoperative range of motion, and lower functional outcomes scores preoperatively are predictive of a slower recovery of active anterior forward flexion after reverse shoulder arthroplasty.[162]
Complications
The first series of reverse shoulder arthroplasty with an at least two years follow-up, confirmed the preliminary results with excellent functional outcome and stable glenoid fixation.[163]Beck S, Patsalis T, Busch A, Dittrich F, Dudda M, Jäger M, Wegner A. A substantial and durable improvement in the long term has been reported.[164][165][166] However, the complexity of this procedure with regards to its singular anatomy and special patient population, is reflected by the large number of reported problems and complications. As defined by Zumstein et al., problems can be defined as intra- or postoperative events that are not likely to affect the patient’s final outcome.[167] This will include hematomas, phlebitis, heterotopic ossification, algodystrophy and will not be part of the treated subjects of this thesis. Complications are defined as any intra- or postoperative events that are likely to have a negative influence on the patient’s final outcome, such as intraoperative cement extravasation, intra- or postoperative fractures, dislocations, infections, neurological lesions, radiographic changes such as glenoid or humeral lucent lines, scapular notching, stress shielding, aseptic loosening, reinterventions (without replacement of the component) or revisions (with replacement of the component).
Radiological Changes
This is the most frequently reported complication after reverse shoulder arthroplasty.{Zumstein, 2011 #1000} We thus conducted a retrospective long-term study (Appendix 6). All the current knowledge of this subject is summarized in this work.Mélis Long-term studies reported their prevalence.mettre etude walch
Impingements
Scapular Notching
Scapular notching is the most frequent radiographic change after a reverse shoulder arthroplasty and has been reported as high as 88%.[168] It was initially described as the result of abutment of the prosthetic metaphysis against the scapular neck with the arm in adduction consequent to humerus medialization. Repetitive contact between polyethylene and bone may result in polyethylene wear debris, chronic inflammation and osteolysis,[169] radiolucency around the glenoid component,[170] loosening of the glenoid component,[171] presence of an inferior bone spur, and ossification in the glenohumeral space.[172]
Scapular notching typically occurs within six months after surgery and appears to stabilize in most cases. The use of an anterosuperior approach, a high position of the baseplate on the glenoid and superior tilting have all been associated with higher rates of notching caused by mechanical impingement with the arm in adduction. Eccentric glenospheres with an inferior offset and glenoid components with a more lateral offset (bony or metal) can reduce the risk of notching.[173][174] Mizuno et al. analyzed the influence of an eccentric glenosphere in 47 consecutive cases compared with an historical group treated by the same surgeon. The rates of notching were not different but the severity of notching was less when using an eccentric glenosphere.[175] Other authors have reported a negligible rate of notching when using an inferior offset component.
Two types of impingement interactions are noted (Figure).
File:Sensitive-content.pngement (Figure C and D).
Anterior Impingement
Anterior impingement can also occur in the setting of reverse shoulder arthroplasty. Anterior impingement may specifically jeopardize the clinical outcome and implant survivorship, ranging from limitation in internal rotation to dislocation by decoaptation, or failure.[176] As the conformation of the joint changes from spinning to hinging in reverse shoulder arthroplasty, implant version of the humeral stem seems the most predictive factor for the occurrence anterior scapular notching. Grammont already warned that excessive retroversion led to decreased internal rotation.[177] The anterior notching mostly occurred in adduction. The best compromise between anterior and posterior notching to favor a functional arc of motion seems to be 20 to 40 degrees of humeral retroversion.[178] On the other hand, glenoid component version doesn’t seem to influence notching in the axial plane.[179]
Infection
The incidence of infections after primary reverse shoulder arthroplasty is around 5%,[16] which is higher than in anatomic shoulder arthroplasty.[180][181] Reasons are the large dead space caused by the ball-and-socket configuration, the frequent postoperative hematoma, the extensive surgical dissection, and in some patients the compromised general health and the numerous previous surgeries. The commonly identified low-virulence organism are Cutibacterium acnes and Staphylococcus epidermidis. Proven and suspected infections should be revised operatively. Acute infection of less than three weeks in a stable arthroplasty should be treated with debridement and antibiotics. Late infections should be treated with arthroplasty removal, debridement and reimplantation.
Instability
Dislocation is one of the most common complications after reverse shoulder arthroplasty, with rates as high as 14% which account for almost half of the complications in some series. Intraoperative criteria have been proposed by other authors to assess prosthetic stability. The recommendations are numerous and include 1) a prosthesis implantation in such a way that it is difficult to reduce, 2) the absence of pistoning of the prosthesis when applying axial traction on the arm, 3) stability throughout a full range of motion, 4) passive adduction of the arm with elbow at side, 5) palpation of the tension in the conjoint tendon after reduction with the arm at the side and the elbow extended, 6) no asymmetric subluxation or tilting of the proximal humeral component on the glenosphere during adduction, and 7) free glenohumeral motion without scapula-thoracic motion between 0° to 60° of abduction. Most cases of dislocation occur during the first few months after implantation and are a result of a technical error. Risk factors for dislocation include body mass index > 30, male sex, previous surgery, subscapularis deficiency and high neck shaft angle (155 degrees). The etiology of dislocation is multifactorial. It can occur due to 1) deltoid insufficiency, 2) lack of anterior restraints including subscapularis insufficiency, conjoint tendon weakness, and pectoralis major insufficiency, 3) malpositioning of the components, 4) impingement, and 5) infection. Instability is more frequent in cases of revision arthroplasty. Deltoid insufficiency can be caused by preoperative factors or can result from a postoperative lack of deltoid tension, acromion fracture, polyethylene wear, stem subsidence, or postoperative neurological palsy. Lädermann et al. noted a strong correlation (p < 0.0001) between preoperative humeral length and dislocation. Postoperative shortening of the humerus, as compared to preoperative or contralateral humeral length, was observed in all cases of dislocation.[182] Subscapularis integrity is important if a 155 degrees is used.[183][184] Low neck shaft angles (145 and 135 degrees) are more stable designs and subscapularis integrity seems less important to prevent instability.
Neurological Lesions
Lengthening of the arm during reverse shoulder arthroplasty, because of its nonanatomic design and/or maneuver of glenohumeral reduction, may be a major factor responsible for the increased prevalence of neurologic injury. Clinically relevant neurological complications involving the brachial plexus or the axillary nerve, however, are rare following reverse shoulder arthroplasty. A prospective study determined the electrodiagnostic occurrence of peripheral nerve lesions following 155 degrees neck shaft angle reverse shoulder arthroplasty.[185] If one also takes into account subclinical deterioration of preoperative lesions, 63% of patients in this study had postoperative neurologic lesions. However, only 5% of patients had a lesion that was present beyond 6 months postoperative. The rate of postoperative lesions seems lower using low neck shaft angles.[186] It seems consequently that distalization put the nerve at risk and that lateralization is rather protective for the plexus.
Glenoid or humeral non- or disassembly
Glenoid or humeral non- or disassembly, and polyethylene disassociation are minor problems and are mainly due to prosthetic design (Figure).
Periprothetic fractures
Humerus
Humeral fractures occurred intra- or postoperatively.
Intraoperative
Intraoperatively, they can appear in the metaphyseal area (“controlled fracture” according to Walch) and are related to retractor positioning. Humeral diaphyseal fractures occur intraoperatively in case of an incorrect sizing of the component or excessive external rotation during preparation of the glenoid and release. They usually require the use of a longer implant to bypass the fracture line or an open reduction internal fixation (ORIF).
Postoperative
Postoperatively, fractures usually result from trauma (Figure). They can be treated either conservatively if the component is stable or they require revision in cases of unstable components.
Heterotopic ossification
Heterotopic ossification after reverse shoulder arthroplasty (Figure) is a relatively common finding of unknown clinical importance.
References
- ↑ Grammont PM, Latfay, J, Deries X. [In French] Etude et réalisation d’une nouvelle prothèse d’épaule. Rhumatologie 1987;39: 407-418
- ↑ Renard JF, Grammont P. [In French] La prothe`se autocentrique de rotule: technique et re´sultats apre`s 7 ans de recul. Rhumatologie. 1989;41:241–245.
- ↑ Guichet JM, Grammont PM, Trouilloud P. [A nail for progressive lengthening. An animal experiment with a 2-year follow-up]. Chirurgie. 1992;118(6-7):405-410
- ↑ Baulot, E. Sirveaux, F. Boileau, P. Grammont's idea: The story of Paul Grammont's functional surgery concept and the development of the reverse principle. Clin Orthop Relat Res. 2011 Sep;469(9):2425-31
- ↑ Inman, VT. Saunders, M. Abbott, MC. Observations on the function of the shoulder joint. J Bone Joint Surg Br 1944;26(1):1-30
- ↑ Pearl, R., and Schultz, F.: Human Biology: A Record of Research. Edited, Baltimore, Warwick and York Inc Publishers, 1930
- ↑ Grammont, P. M.: Place de l’ostéotomie de l’épine de l’omoplate avec translation, rotation, élévation de l’acromion dans les ruptures chroniques de la coiffe des rotateurs. Lyon Chir 1979;55:327–329
- ↑ Neer, C. S., 2nd; Craig, E. V.; and Fukuda, H.: Cuff-tear arthropathy. J Bone Joint Surg Am 1983;65(9):1232-44
- ↑ Sanchez-Sotelo, J.; Cofield, R. H.; and Rowland, C. M.: Shoulder hemiarthroplasty for glenohumeral arthritis associated with severe rotator cuff deficiency. J Bone Joint Surg Am 2001;83(12):1814-22
- ↑ Barrett, W. P.; Franklin, J. L.; Jackins, S. E.; Wyss, C. R.; and Matsen, F. A., 3rd: Total shoulder arthroplasty. J Bone Joint Surg Am 1987;69(6):865-72
- ↑ Grammont, P. M.: Place de l’ostéotomie de l’épine de l’omoplate avec translation, rotation, élévation de l’acromion dans les ruptures chroniques de la coiffe des rotateurs. Lyon Chir 1979;55:327–329
- ↑ Grammont, P. M.; Bourgon, J.; and Pelzer, P.: Study of a Mechanical Model for a Shoulder Total Prosthesis: Realization of a Prototype. In ECAM de Lyon. Edited, Dijon, Université Dijon, 1981
- ↑ Gérard, Y.; Leblanc, J. P.; and Rousseau, B.: A complete shoulder prosthesis. Chirurgie 1973;99:655–663
- ↑ Kolbel, R., and Friedebold, G.: [Shoulder joint replacement]. Arch Orthop Unfallchir 1973;76(1):31-9
- ↑ Baulot, E.; Chabernaud, D.; and Grammont, P. M.: [Results of Grammont's inverted prosthesis in omarthritis associated with major cuff destruction. Apropos of 16 cases]. Acta Orthop Belg 1995;61(Suppl 1):112-9
- ↑ 16.0 16.1 Farshad M, Gerber C. Reverse total shoulder arthroplasty-from the most to the least common complication. Int Orthop. 2010 Dec;34(8):1075-82
- ↑ Terrier A, Reist A, Merlini F, Farron A. Simulated joint and muscle forces in reversed and anatomic shoulder prostheses. J Bone Joint Surg Br 2008;90:751-6.
- ↑ 18.0 18.1 Collin P, Liu X, Denard PJ, Gain S, Nowak A, Lädermann A. Standard versus bony increased-offset reverse shoulder arthroplasty: a retrospective comparative cohort study. J Shoulder Elbow Surg. 2018;27(1):59-64
- ↑ Melis B, DeFranco M, Lädermann A, Molé D, Favard L, Nérot C, Maynou C, Walch G. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J Bone Joint Surg Br. 2011 Sep;93(9):1240-6
- ↑ Lädermann A, Edwards TB, Walch G. Arm lengthening after reverse shoulder arthroplasty: a review. Int orthop 2014;38:991-1000
- ↑ Gagey O, Hue E. Mechanics of the deltoid muscle. A new approach. Clin Orthop Relat Res 2000:250-7
- ↑ Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg 2005;14:147S-61S
- ↑ Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg 2005;14:147S-61S
- ↑ Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: The Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg 2006;15:527-40
- ↑ Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg 2005;14:147S-61S
- ↑ Lädermann A, Edwards TB, Walch G. Arm lengthening after reverse shoulder arthroplasty: a review. Int Orthop. 2014 May;38(5):991-1000
- ↑ De Biase CF, Ziveri G, Delcogliano M, de Caro F, Gumina S, Borroni M, Castagna A, Postacchini R.The use of an eccentric glenosphere compared with a concentric glenosphere in reverse total shoulder arthroplasty: two-year minimum follow-up results. Int Orthop. 2013 Oct;37(10):1949-55
- ↑ 28.0 28.1 Lädermann A, Tay E, Collin P, Piotton S, Chiu CH, Michelet A, Charbonnier C. Effect of critical shoulder angle, glenoid lateralization, and humeral inclination on range of movement in reverse shoulder arthroplasty. Bone Joint Res. 2019 Sep 3;8(8):378-386
- ↑ Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011 Sep;469(9):2512-20
- ↑ Melis B, DeFranco M, Lädermann A, Molé D, Favard L, Nérot C, Maynou C, Walch G.An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J Bone Joint Surg Br. 2011 Sep;93(9):1240-6
- ↑ Mizuno N, Denard PJ, Raiss P, Walch G. The clinical and radiographical results of reverse total shoulder arthroplasty with eccentric glenosphere. Int Orthop. 2012 Aug;36(8):1647-53
- ↑ Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg 2005;14:524-8.
- ↑ Lädermann A, Edwards TB, Walch G. Arm lengthening after reverse shoulder arthroplasty: a review. Int Orthop. 2014 May;38(5):991-1000
- ↑ Lädermann A, Walch G, Lubbeke A, Drake GN, Melis B, Bacle G, Collin P, Edwards TB, Sirveaux F. Influence of arm lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2012 Mar;21(3):336-41
- ↑ Lädermann A1, Walch G, Lubbeke A, Drake GN, Melis B, Bacle G, Collin P, Edwards TB, Sirveaux F. Influence of arm lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2012 Mar;21(3):336-41
- ↑ Lädermann A1, Walch G, Lubbeke A, Drake GN, Melis B, Bacle G, Collin P, Edwards TB, Sirveaux F. Influence of arm lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2012 Mar;21(3):336-41
- ↑ Boutsiadis A, Lenoir H, Denard PJ, Panisset JC, Brossard P, Delsol P, Guichard F, Barth J. The lateralization and distalization shoulder angles are important determinants of clinical outcomes in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2018 Jul;27(7):1226-1234
- ↑ Lädermann A, Williams MD, Melis B, Hoffmeyer P, Walch G. Objective evaluation of lengthening in reverse shoulder arthroplasty.J Shoulder Elbow Surg. 2009 Jul-Aug;18(4):588-95
- ↑ Lädermann A, Walch G, Lubbeke A, Drake GN, Melis B, Bacle G, Collin P, Edwards TB, Sirveaux F. Influence of arm lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2012 Mar;21(3):336-41
- ↑ Iannotti JP, Walker K, Rodriguez E, Patterson TE, Jun BJ, Ricchetti ET. Accuracy of 3-Dimensional Planning, Implant Templating, and Patient-Specific Instrumentation in Anatomic Total Shoulder Arthroplasty. J Bone Joint Surg Am. 2019 Mar 6;101(5):446-457
- ↑ Walch G, Vezeridis PS, Boileau P, Deransart P, Chaoui J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elbow Surg. 2015 Feb;24(2):302-9
- ↑ Lädermann A, Williams MD, Melis B, Hoffmeyer P, Walch G. Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009 Jul-Aug;18(4):588-95
- ↑ Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg 2005;14:147S-61S
- ↑ 44.0 44.1 Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg 2009;17:284-95
- ↑ Boileau P, Moineau G, Roussanne Y, O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res 2011;469:2558-67
- ↑ Gutierrez S, Levy JC, Frankle MA, et al. Evaluation of abduction range of motion and avoidance of inferior scapular impingement in a reverse shoulder model. J Shoulder Elbow Surg 2008;17:608-15
- ↑ Frankle MA, Teramoto A, Luo ZP, Levy JC, Pupello D. Glenoid morphology in reverse shoulder arthroplasty: classification and surgical implications. J Shoulder Elbow Surg 2009;18:874-85
- ↑ Jobin CM, Brown GD, Bahu MJ, et al. Reverse total shoulder arthroplasty for cuff tear arthropathy: the clinical effect of deltoid lengthening and center of rotation medialization. J Shoulder Elbow Surg 2012;21:1269-77
- ↑ Chacon A, Virani N, Shannon R, Levy JC, Pupello D, Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009 Jan;91(1):119-27
- ↑ Lädermann A, Walch G, Lubbeke A, Drake GN, Melis B, Bacle G, Collin P, Edwards TB, Sirveaux F. Influence of arm lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2012 Mar;21(3):336-41
- ↑ Lädermann A, Williams MD, Mélis B, Hoffmeyer P, Walch G. Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2009;18:588-95
- ↑ Lädermann A, Denard PJ, Collin P, Zbinden O, Chiu JC, Boileau P, Olivier F, Walch G.Effect of humeral stem and glenosphere designs on range of motion and muscle length in reverse shoulder arthroplasty. Int Orthop. 2020 Jan 3. doi: 10.1007/s00264-019-04463-2. [Epub ahead of print]
- ↑ 53.0 53.1 Lädermann A, Tay E, Collin P, Piotton S, Chiu CH, Michelet A, Charbonnier C. Effect of critical shoulder angle, glenoid lateralization, and humeral inclination on range of movement in reverse shoulder arthroplasty. Bone Joint Res. 2019 Sep 3;8(8):378-386
- ↑ Lädermann A, Collin P, Denard PJ. Range of motion after reverse shoulder arthroplasty: Which combinations of humeral stem and glenosphere work the best? Obere Extremität 2020 doi:10.1007/s11678-020-00599-5
- ↑ Lädermann A, Denard PJ, Collin P, Zbinden O, Chiu JC, Boileau P, Olivier F, Walch G. Effect of humeral stem and glenosphere designs on range of motion and muscle length in reverse shoulder arthroplasty. Int Orthop. 2020;44(3):519-30
- ↑ 56.0 56.1 Mélis B, Defranco M, Lädermann A, Molé D, Favard L, Nérot C, Maynou C, Walch G. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J Bone Joint Surg Br 2011;93:1240-1246
- ↑ Chae J, Siljander M, Wiater JM. Instability in Reverse Total Shoulder Arthroplasty. J Am Acad Orthop Surg 2018;26:587-596
- ↑ Haidamous G, Lädermann A, Frankle M et al. The risk of postoperative scapular spine fracture following reverse shoulder arthroplasty is increased with an onlay humeral stem. J Shoulder Elbow Surg. 2020;9:S1058-2746(20)30337-2. doi: 10.1016/j.jse.2020.03.036
- ↑ Haidamous G, Lädermann A, Hartzler R et al. Radiographic Parameters Associated With Excellent Versus Poor Range Of Motion Outcomes Following Reverse Shoulder Arthroplasty. Shoulder & Elbow 2020;9:1758573220936234 doi.org/10.1177/1758573220936234
- ↑ Lädermann A, Denard PJ, Tirefort J et al. Subscapularis- and deltoid-sparing vs traditional deltopectoral approach in reverse shoulder arthroplasty: a prospective case-control study. J Orthop Surg Res 2017;12:112
- ↑ Lädermann A, Schwitzguebel AJ, Edwards TB et al. Glenoid loosening and migration in reverse shoulder arthroplasty. Bone Joint J. 2019;101-B:461-469
- ↑ Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg 2009;17:284-295
- ↑ Boileau P, Moineau G, Roussanne Y et al. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res 2011;469:2558-2567
- ↑ 64.0 64.1 Gutierrez S, Comiskey CaT, Luo ZP et al. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. Hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am 2008;90:2606-2615
- ↑ Pauzenberger L, Dwyer C, Obopilwe E, Nowak MD, Cote M, Romeo AA, Mazzocca AD, Dyrna F. Influence of glenosphere and baseplate parameters on glenoid bone strains in reverse shoulder arthroplasty. BMC musculoskeletal disorders 2019;20(1):587
- ↑ Lawrence C, Williams GR, Namdari S. Influence of Glenosphere Design on Outcomes and Complications of Reverse Arthroplasty: A Systematic Review. Clinics in orthopedic surgery 2016;8:288-297
- ↑ Werthel JD, Walch G, Vegehan E, Deransart P, Sanchez-Sotelo J, Valenti P. Lateralization in reverse shoulder arthroplasty: a descriptive analysis of different implants in current practice. Int Orthop 2019;43:2349-2360
- ↑ Boileau P, Moineau G, Roussanne Y et al. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res 2011;469:2558-2567
- ↑ Ernstbrunner L, Werthel JD, Wagner E et al. Glenoid bone grafting in primary reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2017;26:1441-1447
- ↑ Klein SM, Dunning P, Mulieri P et al. Effects of acquired glenoid bone defects on surgical technique and clinical outcomes in reverse shoulder arthroplasty. J Bone Joint Surg Am 2010;92:1144-1154
- ↑ Gutierrez S, Levy JC, Frankle MA et al. Evaluation of abduction range of motion and avoidance of inferior scapular impingement in a reverse shoulder model. J Shoulder Elbow Surg 2008;17:608-615
- ↑ Kim SJ, Jang SW, Jung KH et al. Analysis of impingement-free range of motion of the glenohumeral joint after reverse total shoulder arthroplasty using three different implant models. Journal of orthopaedic science : official journal of the Japanese Orthopaedic Association 2019;24:87-94
- ↑ Lädermann A, Tay E, Collin P et al. Effect of critical shoulder angle, glenoid lateralization, and humeral inclination on range of movement in reverse shoulder arthroplasty. Bone Joint Res 2019;8:378-386
- ↑ Ferle M, Pastor MF, Hagenah J et al. Effect of the humeral neck-shaft angle and glenosphere lateralization on stability of reverse shoulder arthroplasty: a cadaveric study. J Shoulder Elbow Surg 2019;28:966-973
- ↑ Gutierrez S, Greiwe RM, Frankle MA et al. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg 2007;16:S9-S12
- ↑ Werner BS, Chaoui J, Walch G. The influence of humeral neck shaft angle and glenoid lateralization on range of motion in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2017;26:1726-1731
- ↑ Helmkamp JK, Bullock GS, Amilo NR et al. (2018) The clinical and radiographic impact of center of rotation lateralization in reverse shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 27:2099-2107
- ↑ Greiner S, Schmidt C, Herrmann S et al. (2015) Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg 24:1397-1404
- ↑ Athwal GS, Macdermid JC, Reddy KM et al. (2015) Does bony increased-offset reverse shoulder arthroplasty decrease scapular notching? J Shoulder Elbow Surg 24:468-473
- ↑ Athwal GS, Macdermid JC, Reddy KM et al. (2015) Does bony increased-offset reverse shoulder arthroplasty decrease scapular notching? J Shoulder Elbow Surg 24:468-473
- ↑ Zitkovsky HS, Carducci MP, Mahendraraj KA, Grubhofer F, Jawa A. Lateralization and Decreased Neck-Shaft Angle Reduces Scapular Notching and Heterotopic Ossification. J Am Acad Orthop Surg. 2020 Apr 8. doi: 10.5435/JAAOS-D-19-00808. Online ahead of print.
- ↑ Kennon JC, Songy C, Bartels D, Statz J, Cofield RH, Sperling JW, Sanchez-Sotelo J. Primary reverse shoulder arthroplasty: how did medialized and glenoid-based lateralized style prostheses compare at 10 years? J Shoulder Elbow Surg. 2020;29(7S):S23-S31
- ↑ De Wilde LF, Poncet D, Middernacht B, Ekelund A.Prosthetic overhang is the most effective way to prevent scapular conflict in a reverse total shoulder prosthesis. Acta Orthop 2010 81:719-726
- ↑ Kolmodin J, Davidson IU, Jun BJ, Sodhi N, Subhas N, Patterson TE, Li ZM, Iannotti JP, Ricchetti ET. Scapular Notching After Reverse Total Shoulder Arthroplasty: Prediction Using Patient-Specific Osseous Anatomy, Implant Location, and Shoulder Motion. J Bone Joint Surg Am. 2018 Jul 5;100(13):1095-1103
- ↑ Jobin CM, Brown GD, Bahu MJ, et al. Reverse total shoulder arthroplasty for cuff tear arthropathy: the clinical effect of deltoid lengthening and center of rotation medialization. J Shoulder Elbow Surg 2012;21:1269-77
- ↑ Lädermann A, Walch G, Lubbeke A, Drake GN, Melis B, Bacle G, Collin P, Edwards TB, Sirveaux F. Influence of arm lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2012 Mar;21(3):336-41
- ↑ Renaud P, Wahab H, Bontoux L, Dauty M, Richard I, Bregeon C. [Total inverted shoulder prosthesis and rotator cuff insufficiency: evaluation and determination of anatomical parameters predictive of good functional outcome in 21 shoulders]. Ann Readapt Med Phys 2001 ;44(5):273-80
- ↑ Iannotti JP, Walker K, Rodriguez E, Patterson TE, Jun BJ, Ricchetti ET. Accuracy of 3-Dimensional Planning, Implant Templating, and Patient-Specific Instrumentation in Anatomic Total Shoulder Arthroplasty. J Bone Joint Surg Am. 2019 Mar 6;101(5):446-457
- ↑ Walch G, Vezeridis PS, Boileau P, Deransart P, Chaoui J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elbow Surg. 2015 Feb;24(2):302-9
- ↑ Black EM, Roberts SM, Siegel E, et al. Reverse shoulder arthroplasty as salvage for failed prior arthroplasty in patients 65 years of age or younger. J Shoulder Elbow Surg 2014;23:1036-42
- ↑ Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: Results after five to fifteen years. J Shoulder Elbow Surg 2013;22:1199-208
- ↑ Muh SJ, Streit JJ, Wanner JP, et al. Early follow-up of reverse total shoulder arthroplasty in patients sixty years of age or younger. J Bone Joint Surg [Am] 2013;95:1877-83
- ↑ Otto RJ, Clark RE, Frankle MA. Reverse shoulder arthroplasty in patients younger than 55 years: 2- to 12-year follow-up. J Shoulder Elbow Surg 2017;26:792-7
- ↑ Sershon RA, Van Thiel GS, Lin EC, et al. Clinical outcomes of reverse total shoulder arthroplasty in patients aged younger than 60 years. J Shoulder Elbow Surg 2014;23:395-400
- ↑ Walters JD, Barkoh K, Smith RA, Azar FM, Throckmorton TW. Younger patients report similar activity levels to older patients after reverse total shoulder arthroplasty. J Shoulder Elbow Surg2016;25:1418-24
- ↑ Smith CD, Guyver P, Bunker TD. Indications for reverse shoulder replacement: A systematic review. J Bone Joint Surg [Br] 2012;94:577-83
- ↑ Lädermann A, Chiu J, Collin P, Piotton S, Nover L, Scheibel M. Hemi- vs reverse shoulder arthroplasty for acute proximal humeral fractures: a systematic review of level I and II studies. Obere Extremität 2019;14:127–35
- ↑ Statz JM, Schoch BS, Sanchez-Sotelo J, Sperling JW, Cofield RH.Shoulder arthroplasty for locked anterior shoulder dislocation: a role for the reversed design. Int Orthop. 2017 Jun;41(6):1227-1234
- ↑ Cho CH, Kim DH, Song KS. Reverse Shoulder Arthroplasty in Patients with Rheumatoid Arthritis: A Systematic Review. Clin Orthop Surg. 2017 Sep;9(3):325-331
- ↑ Gauci MO, Cavalier M, Gonzalez JF, Holzer N, Baring T, Walch G, Boileau P. Revision of failed shoulder arthroplasty: epidemiology, etiology, and surgical options. J Shoulder Elbow Surg. 2019 Oct 6. pii: S1058-2746(19)30531-2
- ↑ Bonnevialle N, Mansat P, Lebon J, Laffosse JM, Bonnevialle P. Reverse shoulder arthroplasty for malignant tumors of proximal humerus. J Shoulder Elbow Surg. 2015 Jan;24(1):36-44.
- ↑ Lädermann A, Walch G, Denard PJ, Collin P, Sirveaux F, Favard L, Edwards TB, Kherad O, Boileau P. Reverse shoulder arthroplasty in patients with pre-operative impairment of the deltoid muscle. Bone Joint J. 2013 Aug;95-B(8):1106-13
- ↑ Lädermann A, Walch G, Denard PJ, Collin P, Sirveaux F, Favard L, Edwards TB, Kherad O, Boileau P. Reverse shoulder arthroplasty in patients with pre-operative impairment of the deltoid muscle. Bone Joint J. 2013 Aug;95-B(8):1106-13
- ↑ Giuseffi SA, Wongtriratanachai P, Omae H, Cil A, Zobitz ME, An KN, Sperling JW, Steinmann SP. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg 2012;21:1087-95.
- ↑ Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am 2005;87(Suppl 2):1-8
- ↑ Molé D, Wein F, Dézaly C, Valenti P, Sirveaux F. Surgical technique: the anterosuperior approach for reverse shoulder arthroplasty. Clin Orthop Relat Res. 2011 Sep;469(9):2461-8
- ↑ Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics 1993;16:65-8
- ↑ Mackenzie D. The antero-superior exposure for total shoulder replacement. Orthop Traumatol 1993;2:71-7
- ↑ Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles. A cadaver study. J Bone Joint Surg Br 1993;75:137-40
- ↑ Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles. A cadaver study. J Bone Joint Surg Br 1993;75:137-40
- ↑ Collin P, Matsumura N, Lädermann A, Denard PJ, Walch G. Relationship between massive chronic rotator cuff tear pattern and loss of active shoulder range of motion. J Shoulder Elbow Surg 2014;23:1195-202
- ↑ Bianchi S, Martinoli C, Abdelwahab IF. Imaging findings of spontaneous detachment of the deltoid muscle as a complication of massive rotator cuff tear. Skeletal radiology 2006;35:410-5
- ↑ Blix M. Die lange und dle spannung des muskels. Skand Arch Physiol 1891:295-318
- ↑ Gazielly D. The deltoid flap procedure. Tech Shoulder Elbow Surg 2000:117–27
- ↑ Glanzmann MC, Flury M, Simmen BR. Reverse shoulder arthroplasty as salvage procedure after deltoid muscle flap transfer for irreparable rotator cuff tear: a case report. J Shoulder Elbow Surg 2009;18:e1-2
- ↑ Tay AK, Collin P. Irreparable spontaneous deltoid rupture in rotator cuff arthropathy: the use of a reverse total shoulder replacement. J Shoulder Elbow Surg 2011;20:e5-8
- ↑ Sher JS, Iannotti JP, Warner JJ, Groff Y, Williams GR. Surgical treatment of postoperative deltoid origin disruption. Clin Orthop Relat Res 1997:93-8
- ↑ Blazar PE, Williams GR, Iannotti JP. Spontaneous detachment of the deltoid muscle origin. J Shoulder Elbow Surg 1998;7:389-92
- ↑ Morisawa K, Yamashita K, Asami A, Nishikawa H, Watanabe H. Spontaneous rupture of the deltoid muscle associated with massive tearing of the rotator cuff. J Shoulder Elbow Surg 1997;6:556-8
- ↑ Chiba D, Sano H, Nakajo S, Fujii F. Traumatic deltoid rupture caused by seatbelt during a traffic accident: a case report. Journal of orthopaedic surgery 2008;16:127-9
- ↑ Lin JT, Nagler W. Partial tear of the posterior deltoid muscle in an elderly woman. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine 2003;13:120-1
- ↑ Wilbourn AJ, Aminoff MJ. AAEM minimonograph 32: the electrodiagnostic examination in patients with radiculopathies. American Association of Electrodiagnostic Medicine. Muscle Nerve 1998:1612-31
- ↑ Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res 1994:78-83
- ↑ Moser T, Lecours J, Michaud J, Bureau NJ, Guillin R, Cardinal E. The deltoid, a forgotten muscle of the shoulder. Skeletal radiology 2013;42:1361-75
- ↑ ädermann A, Walch G, Denard PJ, et al. Reverse shoulder arthroplasty in patients with pre-operative impairment of the deltoid muscle. The bone & joint journal 2013;95-B:1106-13
- ↑ Glanzmann MC, Flury M, Simmen BR. Reverse shoulder arthroplasty as salvage procedure after deltoid muscle flap transfer for irreparable rotator cuff tear: a case report. J Shoulder Elbow Surg 2009;18:e1-2
- ↑ Tay AK, Collin P. Irreparable spontaneous deltoid rupture in rotator cuff arthropathy: the use of a reverse total shoulder replacement. J Shoulder Elbow Surg 2011;20:e5-8
- ↑ Gulotta LV, Choi D, Marinello P, Wright T, Cordasco FA, Craig EV, Warren RF. Anterior deltoid deficiency in reverse total shoulder replacement: a biomechanical study with cadavers. J Bone Joint Surg Br 2012;94:1666-9
- ↑ Whatley AN, Fowler RL, Warner JJ, Higgins LD. Postoperative rupture of the anterolateral deltoid muscle following reverse total shoulder arthroplasty in patients who have undergone open rotator cuff repair. J Shoulder Elbow Surg 2011;20:114-22
- ↑ Essilfie A, McKnight B, Heckmann N, Rick Hatch GF, 3rd, Omid R. Revision reverse total shoulder arthroplasty in a patient with preoperative deltoid insufficiency: a case report. J Shoulder Elbow Surg 2017;26:e232-e5
- ↑ Dosari M, Hameed S, Mukhtar K, Elmhiregh A. Reverse shoulder arthroplasty for deltoid-deficient shoulder following latissimus dorsi flap transfer. Case report. Int J Surg Case Rep 2017;39:256-9
- ↑ Goel DP, Ross DC, Drosdowech DS. Rotator cuff tear arthropathy and deltoid avulsion treated with reverse total shoulder arthroplasty and latissimus dorsi transfer: case report and review of the literature. J Shoulder Elbow Surg 2012;21:e1-7
- ↑ Dosari M, Hameed S, Mukhtar K, Elmhiregh A. Reverse shoulder arthroplasty for deltoid-deficient shoulder following latissimus dorsi flap transfer. Case report. Int J Surg Case Rep 2017;39:256-9
- ↑ Marinello PG, Amini MH, Peers S, O'Donnell J, Iannotti JP. Reverse total shoulder arthroplasty with combined deltoid reconstruction in patients with anterior and/or middle deltoid tears. J Shoulder Elbow Surg 2016;25:936-41
- ↑ Marinello PG, Amini MH, Peers S, O'Donnell J, Iannotti JP. Reverse total shoulder arthroplasty with combined deltoid reconstruction in patients with anterior and/or middle deltoid tears. J Shoulder Elbow Surg 2016;25:936-41
- ↑ Lädermann A, Walch G, Denard PJ, et al. Reverse shoulder arthroplasty in patients with pre-operative impairment of the deltoid muscle. The bone & joint journal 2013;95-B:1106-13
- ↑ Lädermann A, Walch G, Denard PJ, et al. Reverse shoulder arthroplasty in patients with pre-operative impairment of the deltoid muscle. The bone & joint journal 2013;95-B:1106-13
- ↑ Schneeberger AG, Muller TM, Steens W, Thur C. Reverse total shoulder arthroplasty after failed deltoid flap reconstruction. Arch Orthop Trauma Surg 2014;134:317-23
- ↑ Crosby LA, Hamilton A, Twiss T. Scapula fractures after reverse total shoulder arthroplasty: classification and treatment. Clin Orthop Relat Res 2011;469:2544-9
- ↑ Otto RJ, Virani NA, Levy JC, Nigro PT, Cuff DJ, Frankle MA. Scapular fractures after reverse shoulder arthroplasty: evaluation of risk factors and the reliability of a proposed classification. J Shoulder Elbow Surg. 2013 Nov;22(11):1514-21
- ↑ Schenk P, Aichmair A, Beeler S, Ernstbrunner L, Meyer DC, Gerber C. Acromial Fractures Following Reverse Total Shoulder Arthroplasty: A Cohort Controlled Analysis. Orthopedics. 2020 Jan 1;43(1):15-22
- ↑ Schenk P, Aichmair A, Beeler S, Ernstbrunner L, Meyer DC, Gerber C. Acromial Fractures Following Reverse Total Shoulder Arthroplasty: A Cohort Controlled Analysis. Orthopedics. 2020 Jan 1;43(1):15-22
- ↑ Wong MT, Langohr GDG, Athwal GS, Johnson JA. Implant positioning in reverse shoulder arthroplasty has an impact on acromial stresses. J Shoulder Elbow Surg. 2016 Nov;25(11):1889-1895
- ↑ Crosby LA, Hamilton A, Twiss T. Scapula fractures after reverse total shoulder arthroplasty: classification and treatment. Clin Orthop Relat Res 2011;469:2544-9
- ↑ Mottier F, Wall B, Nove-Josserand L, Galoisy Guibal L, Walch G. [Reverse prosthesis and os acromiale or acromion stress fracture]. Rev Chir Orthop Reparatrice Appar Mot 2007;93:133-41
- ↑ Neyton L, Erickson J, Ascione F, Bugelli G, Lunini E, Walch G. Grammont Award 2018: Scapular fractures in reverse shoulder arthroplasty (Grammont style): prevalence, functional, and radiographic results with minimum 5-year follow-up. J Shoulder Elbow Surg. 2019 Feb;28(2):260-267
- ↑ Crosby LA, Hamilton A, Twiss T. Scapula fractures after reverse total shoulder arthroplasty: classification and treatment. Clin Orthop Relat Res 2011;469:2544-9
- ↑ Wall B, Nove-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am 2007;89:1476-85
- ↑ Wall B, Nove-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am 2007;89:1476-85
- ↑ Hartzler RU, Steen BM, Hussey MM, Cusick MC, Cottrell BJ, Clark RE, Frankle MA. Reverse shoulder arthroplasty for massive rotator cuff tear: risk factors for poor functional improvement. J Shoulder Elbow Surg 2015;24:1698-706
- ↑ Schwartz DG, Cottrell BJ, Teusink MJ, Clark RE, Downes KL, Tannenbaum RS, Frankle MA. Factors that predict postoperative motion in patients treated with reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014 Sep;23(9):1289-95
- ↑ Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon's experience? J Shoulder Elbow Surg. 2012 Nov;21(11):1470-7
- ↑ Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am 2008;90:1244-51.
- ↑ Wall B, Nove-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am 2007;89:1476-85
- ↑ Lädermann A, Walch G, Denard PJ, et al. Reverse shoulder arthroplasty in patients with pre-operative impairment of the deltoid muscle. The bone & joint journal 2013;95-B:1106-13
- ↑ Schwartz DG, Cottrell BJ, Teusink MJ, Clark RE, Downes KL, Tannenbaum RS, Frankle MA. Factors that predict postoperative motion in patients treated with reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014 Sep;23(9):1289-95
- ↑ Lädermann A, Edwards TB, Walch G. Arm lengthening after reverse shoulder arthroplasty: a review. International orthopaedics 2014;38:991-1000
- ↑ Lädermann A, Lubbeke A, Collin P, Edwards TB, Sirveaux F, Walch G. Influence of surgical approach on functional outcome in reverse shoulder arthroplasty. Orthopaedics & traumatology, surgery & research : OTSR 2011;97:579-82
- ↑ Lädermann A, Williams MD, Mélis B, Hoffmeyer P, Walch G. Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2009;18:588-95
- ↑ Hartzler RU, Steen BM, Hussey MM, Cusick MC, Cottrell BJ, Clark RE, Frankle MA. Reverse shoulder arthroplasty for massive rotator cuff tear: risk factors for poor functional improvement. J Shoulder Elbow Surg 2015;24:1698-706
- ↑ Lädermann A, Lubbeke A, Melis B, et al. Prevalence of neurologic lesions after total shoulder arthroplasty. J Bone Joint Surg Am 2011;93:1288-93
- ↑ Collin P, Matsukawa T, Denard PJ, Gain S, Lädermann A.Pre-operative factors influence the recovery of range of motion following reverse shoulder arthroplasty. Int Orthop. 2017 Oct;41(10):2135-2142
- ↑ Baulot E, Chabernaud D, Grammont PM. [Results of Grammont's inverted prosthesis in omarthritis associated with major cuff destruction. Apropos of 16 cases]. Acta Orthop Belg 1995;61 Suppl 1:112-9
- ↑ Long-term results of the reverse Total Evolutive Shoulder System (TESS). Arch Orthop Trauma Surg. 2019 Aug;139(8):1039-1044
- ↑ Ernstbrunner L, Rahm S, Suter A, Imam MA, Catanzaro S, Grubhofer F, Gerber C. Salvage reverse total shoulder arthroplasty for failed operative treatment of proximal humeral fractures in patients younger than 60 years: long-term results. J Shoulder Elbow Surg. 2019 Oct 6. pii: S1058-2746(19)30537-3. doi: 10.1016/j.jse.2019.07.040. [Epub ahead of print]
- ↑ van Ochten JHM, van der Pluijm M, Pouw M, Felsch QTM, Heesterbeek P, de Vos MJ. Long - Term survivorship and clinical and radiological follow - up of the primary uncemented Delta III reverse shoulder prosthesis. J Orthop. 2019 Mar 24;16(4):342-346
- ↑ Zumstein MA1, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011 Jan;20(1):146-57
- ↑ Mélis B, DeFranco M, Lädermann A, Molé D, Favard L, Nérot C, Maynou C, Walch G. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J Bone Joint Surg Br 2011;93:1240-6
- ↑ Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg 2005;14:524-8
- ↑ Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am 2005;87:1476-86
- ↑ Cazeneuve JF, Cristofari DJ. Delta III reverse shoulder arthroplasty: radiological outcome for acute complex fractures of the proximal humerus in elderly patients. OOrthop Traumatol Surg Res 2009;95:325-9
- ↑ Mélis B, DeFranco M, Lädermann A, Molé D, Favard L, Nérot C, Maynou C, Walch G. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J Bone Joint Surg Br 2011;93:1240-6
- ↑ Lädermann A, Denard PJ, Collin P, Zbinden O, Chiu JC, Boileau P, Olivier F, Walch G. Effect of humeral stem and glenosphere designs on range of motion and muscle length in reverse shoulder arthroplasty. Int Orthop. 2020 Jan 3. doi: 10.1007/s00264-019-04463-2. [Epub ahead of print]
- ↑ Lädermann A, Tay E, Collin P, Piotton S, Chiu CH, Michelet A, Charbonnier C. Effect of critical shoulder angle, glenoid lateralization, and humeral inclination on range of movement in reverse shoulder arthroplasty. Bone Joint Res. 2019 Sep 3;8(8):378-386
- ↑ Mizuno N, Denard PJ, Raiss P, Walch G. The clinical and radiographical results of reverse total shoulder arthroplasty with eccentric glenosphere. Int Orthop. 2012 Aug;36(8):1647-53
- ↑ De Wilde L1, Walch G. Humeral prosthetic failure of reversed total shoulder arthroplasty: a report of three cases. J Shoulder Elbow Surg. 2006 Mar-Apr;15(2):260-4
- ↑ Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics 1993;16:65-8
- ↑ Stephenson DR, Oh JH, McGarry MH, Rick Hatch GF 3rd, Lee TQ. Effect of humeral component version on impingement in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011 Jun;20(4):652-8
- ↑ Favre P, Sussmann PS, Gerber C. The effect of component positioning on intrinsic stability of the reverse shoulder arthroplasty. J Shoulder Elbow Surg 2010;19:550–556
- ↑ ehringer EV, Mikuls TR, Michaud KD, Henderson WG, O'Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop Relat Res 2010;468:717-22
- ↑ Seebauer L. Total reverse shoulder arthroplasty: European lessons and future trends. Am J Orthop (Belle Mead NJ). 2007 Dec;36(12 Suppl 1):22-8
- ↑ Lädermann A, Williams MD, Melis B, Hoffmeyer P, Walch G. Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009 Jul-Aug;18(4):588-95
- ↑ Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O'Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2009;18:892-6
- ↑ Gallo RA, Gamradt SC, Mattern CJ, et al. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg 2011;20:584-90
- ↑ Lädermann A1, Lübbeke A, Mélis B, Stern R, Christofilopoulos P, Bacle G, Walch G. Prevalence of neurologic lesions after total shoulder arthroplasty. J Bone Joint Surg Am. 2011 Jul 20;93(14):1288-93
- ↑ Lowe JT, Lawler SM, Testa EJ, Jawa A. Lateralization of the glenosphere in reverse shoulder arthroplasty decreases arm lengthening and demonstrates comparable risk of nerve injury compared with anatomic arthroplasty: a prospective cohort study. J Shoulder Elbow Surg. 2018 Oct;27(10):1845-1851